Paraneoplastic Neurologic Syndromes
Josep Dalmau and Myrna R. Rosenfeld
• The paraneoplastic neurologic syndromes include an extensive group of disorders that can affect any part of the central or peripheral nervous system.
• Evidence indicates that many of these syndromes have an immunopathogenesis.
• Patients with paraneoplastic neurologic syndromes may have serum and cerebrospinal fluid antibodies that are highly specific for the presence of a cancer. The detection of these antibodies serves as a marker of paraneoplasia. Other antibodies associate with specific neurologic syndromes that occur with or without cancer. In these cases, the antibodies serve as markers for the neurologic syndrome but do not distinguish between a paraneoplastic or nonparaneoplastic etiology.
• Antibodies directly mediate some disorders such as the Lambert-Eaton myasthenic syndrome, myasthenia gravis, and neuromyotonia and likely mediate other disorders such as anti-N-methyl-d-aspartate receptor encephalitis. In these disorders the antibodies target neuronal cell surface proteins (e.g., receptors and ion channels), and immunotherapy along with tumor treatment often result in substantial neurologic improvement.
• For paraneoplastic neurologic syndromes in which T-cell mechanisms are predominant, the associated antibodies target intracellular neuronal proteins. In this group of disorders, the response to therapy is, in general, disappointing. The physician’s main concern should be to rule out other diagnostic entities and to uncover the presence of the associated neoplasm. For these syndromes the treatment approach should be aimed at the tumor, because stabilization and, less often, improvement of neurologic symptoms after tumor treatment have been reported for almost all syndromes. In a few cases, depending on the syndrome and whether the patient is in the early stages of the neurologic disease, treatment with immunosuppression may have some effect on the paraneoplastic neurologic syndrome.
Introduction
Most paraneoplastic neurologic syndromes are thought to have an immunopathogenesis. One hypothesis is that the expression of neuronal antigens by the tumor triggers the production of antineuronal antibodies that can be found in serum and cerebrospinal fluid (CSF). Some antibodies are highly specific for the paraneoplastic origin of the neurologic symptoms and are not found (or are rarely found at low titers) in patients with similar neurologic disorders without cancer. These antibodies serve as markers of the paraneoplastic origin of neurologic symptoms and as markers for the presence of specific tumor types (Table 39-1).
Table 39-1
Immune Associations in Paraneoplastic Neurologic Disorders
| Antibody | Syndrome | Most Common Tumor Association |
| Anti-Hu | PEM/PSN | SCLC |
| Anti-Yo | PCD | Ovary, breast |
| Anti-Ma | Brainstem encephalitis/PCD | Several |
| Anti-Ma2 | Limbic/brainstem encephalitis | Testicular |
| Anti-Ri | Opsoclonus-ataxia | Breast, gynecologic |
| Anti-Tr | PCD | Hodgkin lymphoma |
| Anti-amphiphysin | Stiff man syndrome | Breast, SCLC |
| Anti-CV2/CRMP5 | PEM/PCD, peripheral neuropathy, uveitis | SCLC, several |
| Anti-recoverin | Retinopathy | SCLC |
| Anti-bipolar cells of retina | Retinopathy | Melanoma |
| Anti-NMDAR* | Limbic encephalitis, seizures, psychiatric symptoms | Teratoma |
| Anti-VGCC* | LEMS, PCD | SCLC |
| Anti-AChR* (muscle) | Myasthenia gravis | Thymoma |
| Anti-AChR* (neuronal) | Autonomic neuropathy | SCLC |
| Anti-Caspr2* | Peripheral nerve hyperexcitability (with/without CNS involvement) | Thymoma, others |
| Anti-LGI1* | Limbic encephalitis | Thymoma, SCLC |
| Anti-AMPAR* | Limbic encephalitis with tendency to relapse | SCLC, thymoma, breast |
| Anti-GABAB* | Limbic encephalitis with prominent seizures | SCLC |
| Anti-mGluR5 | Limbic encephalitis | Hodgkin lymphoma |
*This antibody may occur with or without a cancer association and is therefore not a marker of paraneoplasia.
An immune-mediated pathogenesis has been demonstrated for several syndromes. Patients with Lambert-Eaton myasthenic syndrome (LEMS) have serum antibodies against voltage-gated calcium channels that are expressed by the tumor, which usually is a small cell lung cancer (SCLC). These antibodies block the entry of calcium necessary for the release of quanta of acetylcholine and result in neuromuscular weakness.1 In paraneoplastic myasthenia gravis, a thymoma triggers an immune response against the acetylcholine receptor at the postsynaptic level of the neuromuscular junction, resulting in weakness and fatigability.2 Evidence supporting an immune pathogenesis in other syndromes includes responses to immunomodulatory therapies and in vitro and in vivo studies demonstrating antineuronal effects of the antibodies.3–7 In all cases where antibodies are or appear to be pathogenic, the target antigens are neuronal cell surface receptors or ion channels or proteins that interact with synaptic proteins. Patients with these antibodies often respond to antibody-depleting immunotherapies in addition to treatment of the underlying tumor.
In contrast, there exists a group of antibodies that are directed against intracellular neuronal antigens. In disorders associated with these antibodies, the pathogenicity appears to be mediated by cytotoxic T cells. Autopsies of patients with these antibodies often demonstrate intense inflammatory infiltrates of mononuclear cells, including CD4+ and CD8+, which predominate in the areas that are symptomatic.8,9 Because of the neuronal destruction caused by the cytotoxic T cells, these disorders tend to be refractory or minimally responsive to treatment.
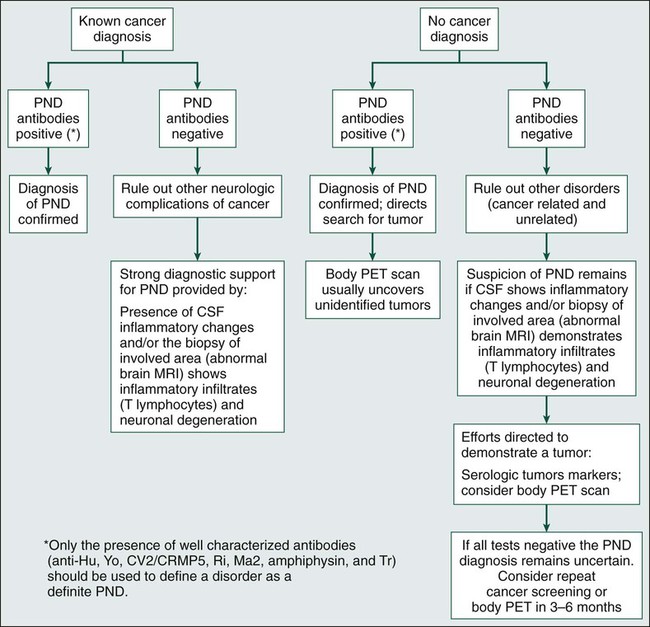
Identification of the paraneoplastic origin of a patient’s symptoms is important because in more than two thirds of patients, the neurologic symptoms develop before the cancer is detected. Because similar disorders may occur without cancer, the diagnosis of the paraneoplastic origin of a disorder depends heavily on the index of suspicion, which is based in part on the syndrome, because some syndromes have a paraneoplastic origin more frequently than do others. For example, the likelihood that LEMS or subacute cerebellar degeneration in a middle-aged or elderly patient is paraneoplastic is probably greater than 50%.10 In contrast, subacute sensory neuropathy or dermatomyositis probably is paraneoplastic in origin in less than 20% of patients.11,12 In most paraneoplastic syndromes, symptoms develop acutely or subacutely and may resemble a viral process. Symptoms evolve over weeks or months and then stabilize, differentiating them from the more chronic and progressive degenerative diseases of middle age and adulthood. Criteria have been proposed to facilitate the diagnosis of paraneoplastic disorders that take into consideration the type of neurologic syndrome, the detection of an associated tumor, and the presence or absence of paraneoplastic antibodies (Box 39-1 and Fig. 39-1).13
Paraneoplastic Syndromes of the Central Nervous System
Paraneoplastic Encephalomyelitis
Patients with PEM experience symptoms of multifocal involvement of the nervous system, resulting in several syndromes that may occur in isolation or in various combinations.14,15 Syndromes include limbic encephalitis, cerebellar degeneration, brainstem encephalitis, myelitis, and sensory and autonomic neuropathies.
Paraneoplastic encephalomyelitis has been described in association with many tumors, but in 70% of patients, the underlying tumor is a SCLC. Most tumors are diagnosed within 2 years of neurologic presentation. Most patients with PEM and SCLC have high titers of anti-Hu antibodies in their serum and CSF, as do some patients with PEM that is associated with other types of cancer.14,16 Antibodies to CV2/CRMP5, with or without anti-Hu antibodies, also occur at a lower frequency and most commonly are associated with thymoma.14,17 A subset of patients with PEM and antibodies to CV2/CRMP5 also experience paraneoplastic chorea and uveitis.18
The onset of symptoms in persons with PEM is subacute. Sensory neuropathy is the most common initial manifestation, followed by brainstem and limbic encephalopathy.14,19,20 Symptom progression is usually relentless, or, less frequently, intermittent, until stabilization. Spontaneous improvement is rare but has been described in patients with limbic encephalitis.21 For most patients the neurologic deficits are severe and incapacitating. Respiratory or autonomic failure due to neurologic dysfunction is commonly the cause of death.14,19
About one third of patients with PEM experience symptoms of cerebellar and/or brainstem dysfunction.14,19 Motor weakness, muscle atrophy, and fasciculations are found in 20% of patients with PEM.19 When spinal cord involvement predominates, a diagnosis of subacute motor neuron dysfunction or atypical motor neuron disease may be entertained until other areas of the nervous system become involved.22,23 Autonomic dysfunction affects about 30% of patients. Symptoms include orthostatic hypotension, gastrointestinal paresis, and pseudoobstruction.24,25 Hypothermia, hypoventilation, sleep apnea, and cardiac arrhythmias may be the cause of sudden death in these patients.
About 75% of patients with anti-Hu–associated PEM experience an asymmetric pansensory neuropathy, called “paraneoplastic sensory neuronopathy” (PSN; see later discussion).14,19 PSN is often associated with painful dysesthesias that result from inflammation of the dorsal root ganglia and neuronal degeneration. For patients in whom motor weakness and a sensory neuropathy develop subacutely, the initial diagnosis may be that of acute inflammatory polyneuritis (Guillain-Barré syndrome [GBS]).26
Limbic Encephalitis
Symptoms of paraneoplastic limbic encephalitis (PLE) may develop alone but more often are found in association with brainstem dysfunction, cerebellar symptoms, or PSN (see “Paraneoplastic Encephalomyelitis”).19 The most common underlying tumor is SCLC, followed by testicular cancer. Regardless of the tumor type, the neurologic dysfunction usually precedes the diagnosis of cancer.
The most characteristic finding is short-term memory deficits with relative preservation of other cognitive functions.27,28 Memory deficits often become evident after several weeks of depression, personality changes, irritability, and seizures. Partial complex temporal lobe seizures with or without motor involvement of the face and extremities are common. Some patients show signs of diencephalic-hypothalamic dysfunction, including drowsiness, hyperthermia, hyperphagia, and, less frequently, pituitary hormonal deficits. The disorder may resemble viral encephalitis or a rapidly developing dementia due to a primary neurodegenerative disorder.
Paraneoplastic limbic encephalitis is one of the few paraneoplastic neurologic disorders in which neuroimaging may be useful. Typical magnetic resonance imaging (MRI) findings include unilateral or bilateral mesial temporal lobe abnormalities best seen on T2-weighted and fluid-attenuated inversion recovery images (Fig. 39-2).21,29–31 On T1-weighted sequences, the temporal-limbic regions may be hypointense and sometimes may enhance with contrast injection. Patients with herpes simplex encephalitis may have similar MRI findings in the early stages of the disease. However, these patients usually exhibit prominent signs of edema and mass effect involving one or both inferomedial temporal lobes, inferior frontal lobes, and the cingulate gyrus, which often is associated with gyral enhancement and signs of hemorrhage.32,33
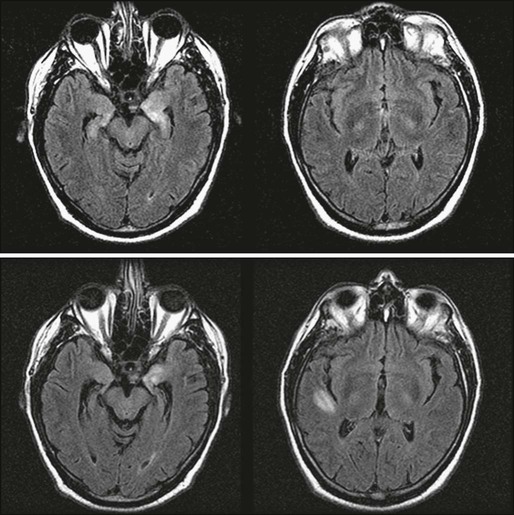
The major pathological findings are seen in the hippocampus, parahippocampal gyrus, cingular cortex, insular cortex, and diencephalon.28 Almost all patients have mild abnormalities in other areas of the nervous system in a pattern of distribution resembling PEM, suggesting that PLE should be regarded as PEM with predominant involvement of the limbic structures.
Antineuronal antibodies, when present in serum and CSF, facilitate the diagnosis of PLE and often allow for early detection of the associated tumor. In patients with SCLC, the anti-Hu antibody is present in about 50% of cases with predominant or isolated symptoms of limbic encephalitis.34 A few patients have been reported with limbic encephalitis and anti-CV2/CRMP5 antibodies. In these patients the underlying tumors are SCLC and thymoma.35 Anti-Ma2 antibodies may be found in the serum and CSF of patients with limbic and/or brainstem encephalopathy. These patients usually have testicular cancer (either seminomatous or nonseminomatous germ cell tumors), but other tumors have been reported.9 Patients with anti-Ma2 antibodies often have additional involvement of the hypothalamus and brainstem and are more likely to have abnormal MRI findings than are other patients with PLE.36
Limbic encephalitis may be associated with antibodies to leucine-rich glioma-inactivated–1 protein (LGI1), an epilepsy-related protein.37 In addition to seizures, some patients experience hyponatremia and rapid eye movement sleep disturbances. The disorder is paraneoplastic in about 20% of cases, with thymoma and SCLC the more commonly associated tumors. Until recently this disorder was incorrectly attributed to antibodies to voltage-gated calcium channels.
Acute limbic dysfunction, sometimes with psychiatric features in association with antibodies to the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, has been reported and appears to more commonly affect middle-aged women.5,38 Approximately 70% of the cases are paraneoplastic, with lung, breast, or thymic tumors being the most common.
Limbic encephalitis associated with antibodies to the gamma-amino-butyric acid type B (GABAB) receptor has been reported.39 Patients with this condition usually have seizures, and in about 50% of cases, patients have SCLC or a neuroendocrine tumor of the lung. Additional antibodies to glutamic acid decarboxylase and antibodies to nonneuronal proteins of unclear significance are frequently present, suggesting a tendency to autoimmunity.40
Two cases of limbic encephalopathy in association with Hodgkin lymphoma (Ophelia syndrome) were reported in which the target antigen was shown to be the metabotropic glutamate receptor 5 (mGluR5).41 One patient responded to corticosteroids and the other to treatment of the tumor. The initial patient reported with Ophelia syndrome also responded to tumor treatment.42
When limbic encephalitis is part of PEM or PSN, it usually responds poorly to treatment. Symptom stabilization or improvement may occur if the tumor is recognized and treated early.9,15,43 A study of patients with SCLC and PLE suggested that the presence of anti-Hu antibodies was associated with a decreased likelihood of improvement.34 In contrast, about 35% of patients with limbic encephalopathy associated with antibodies to Ma2 improve with immunotherapy and treatment of the tumor (of these 35% of patients, most have a testicular germ-cell tumor).44
The syndromes associated with LGI1, AMPA receptor, and GABAB receptor antibodies often respond to treatment of the tumor, when present, and immunotherapy. Patients with AMPA receptor antibodies have a tendency to relapse that can be successfully treated with reinstitution of immunotherapy.5
Anti–N-methyl-D-aspartate Receptor Encephalitis
Anti–N-methyl-d-aspartate (NMDA) receptor encephalitis is the most common of the recently described immune-mediated encephalitides that associate with and are likely caused by antibodies to neuronal cell surface receptors or ion channels (see “Limbic Encephalitis”).45,46 Anti-NMDA receptor encephalitis usually occurs in young women and children of both sexes, although older men and boys may be affected.47 Patients present with an acute change in behavior and personality and memory loss, and they may be diagnosed with a psychiatric disorder or be suspected of malingering or drug abuse. Symptoms progress within days or weeks to include seizures, a decreased level of consciousness, abnormal movements (e.g., orofacial and limb dyskinesias and dystonia), and autonomic instability with frequent hypoventilation.47 The antibodies are directed against the NR1 subunit of the NMDA receptor that is highly enriched in the hippocampus (Fig. 39-3). Studies in vitro and in vivo have shown that the binding of the antibodies to the receptors alter the number and function of the receptors in a reversible manner.6 Titers of antibodies are higher in CSF than in serum, and CSF titers may remain elevated even when serum is negative.
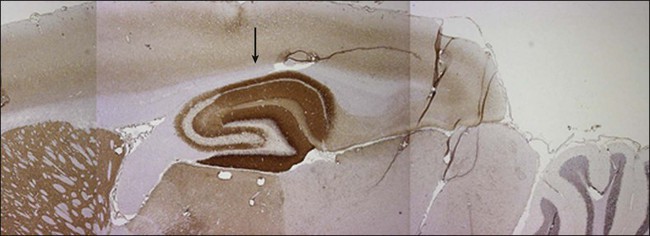
About half of women older than 12 years will have unilateral or bilateral ovarian teratomas (either benign or malignant) that may be mistaken for benign ovarian cysts upon imaging, whereas only 8% of girls younger than 12 years have an associated tumor.48 Tumors occur in only 5% of male patients (usually a testicular germ cell tumor).47
The course of the disorder may be prolonged even with aggressive treatment. Early institution of therapy improves outcome, although patients with prolonged courses will often have substantial if not complete recovery with continued care.47,49 Treatment should focus on identification and removal of a tumor if present and immunotherapy with corticosteroids, intravenous immunoglobulins (IVIGs), or plasma exchange. Patients who do not respond to initial treatments should receive second-line therapy with cyclophosphamide with or without rituximab, because this treatment significantly improves outcomes.48 Relapses occur more frequently in nonparaneoplastic cases and in paraneoplastic cases in which the tumor was not removed.50
Paraneoplastic Cerebellar Degeneration
PCD is characterized by the subacute development of rapidly progressive cerebellar dysfunction, which stabilizes after a few months, leaving the patient severely disabled.51 Postmortem studies demonstrate near or total loss of Purkinje cells with relative preservation of other cerebellar neurons, and they also demonstrate Bergmann astrogliosis (Fig. 39-4). Almost every type of tumor has been reported in association with PCD; the most common neoplasms are gynecologic tumors, breast and lung cancers, and lymphomas.52,53 Neurologic symptoms precede detection of the tumor in about 60% of patients.
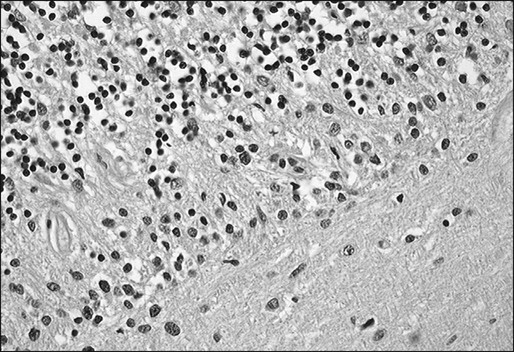
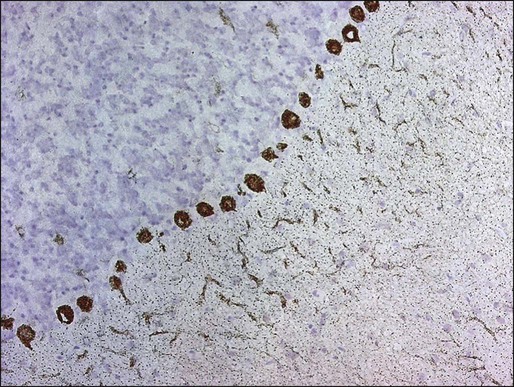
Manifesting symptoms of PCD include dizziness, visual problems, nausea, vomiting, and dysarthria. Within days or even hours, ataxia of gait and of the extremities develops, usually accompanied by dysphagia. Some patients with PCD who have gynecologic or breast tumors have serum and CSF antibodies called anti-Yo that react with 34- and 62-kd proteins expressed by Purkinje cells and the underlying tumor.51 Other patients may have PCD in association with Hodgkin disease, in which case an antibody called anti-Tr usually is found (Fig. 39-5).54,55
For patients with SCLC, PCD may develop in association with LEMS.52,56 Because LEMS symptoms often are treatable, suspicion of LEMS should prompt an appropriate investigation with electrophysiological testing or measurement of antibodies directed against P/Q type voltage-gated calcium channels. These antibodies may also be present in some patients with SCLC and PCD without symptoms of LEMS.57
A distinctive clinical syndrome occurring predominantly in women is characterized by the subacute onset of ataxia and opsoclonus.58 The ataxia predominates in the trunk, causing severe gait difficulty and frequent falls. In half of these patients, the neurologic symptoms develop before the tumor is diagnosed. The tumor usually is a breast cancer or, less frequently, gynecologic cancers or SCLC. The serum and CSF of these patients contain an antibody called anti-Ri, which is expressed by neurons and the associated tumor.58,59
In patients with SCLC, the development of PCD may be the manifesting symptom of PEM. In such cases, anti-Hu or anti-CV2/CRMP5 antibodies usually are present, and patients eventually develop signs and symptoms of multifocal neurologic disease. Patients whose symptoms remain restricted to the cerebellum and who do not harbor anti-Hu antibodies may have antibodies to voltage-gated calcium channels.60
In one subset of patients, PCD develops in the setting of brainstem dysfunction. These patients have serum and spinal fluid antibodies against Ma1 and Ma2 proteins expressed in neurons and spermatogenic cells of the testis. These patients have a variety of associated cancers, including those of the lung, breast, parotid, colon, and testis.61
Most patients with PCD do not improve, although there are isolated reports of improvement with treatment of the tumor, corticosteroids, IVIG, plasma exchange, rituximab, or cyclophosphamide.64–64
Motor Neuron Syndromes
The existence of paraneoplastic motor neuron dysfunction is based on reports of patients with typical amyotrophic lateral sclerosis (ALS) who improved after treatment of the underlying tumor, suggesting more than a coincidental relationship.65,66 In patients with cancer and symptoms of motor neuron disease, the most common neoplasms are carcinoma of the lung, breast, kidney, and lymphoma.67,68 For these patients, the neurologic syndrome and laboratory studies are similar to those seen in patients with typical ALS.
Patients with PEM may experience symptoms resembling motor neuron disease.19,22 These patients almost always exhibit signs of involvement of other areas of the nervous system, which, along with the presence of the anti-Hu antibody, helps to rule out ALS.
Patients with Hodgkin and non-Hodgkin lymphoma may experience a subacute lower motor neuronopathy.69 Typically, these patients have subacute, progressive, painless, and asymmetric involvement of the extremities, with legs affected more than the arms. In contrast to typical ALS, fasciculations are rare, bulbar muscles are usually spared, and upper motor neuron signs are absent. Examination of the CSF is normal or shows mildly increased proteins, and electrophysiological studies demonstrate denervation with normal or mild slowing of motor nerve conduction velocities. Neurologic stabilization or spontaneous improvement may occur. Paraneoplastic subacute lower motor neuronopathy must be distinguished from the lower motor neuron dysfunction that may develop after radiation therapy of the spinal cord.70
Stiff Man Syndrome
Stiff man syndrome is an unusual disorder characterized by progressive muscle stiffness, aching, muscle spasms, and rigidity. The stiffness develops over a period of months and is most prominent in the paraspinal muscles and lower limbs. The muscle spasms are painful and triggered by a variety of stimuli. They are severe enough to produce limb deformities and fractures.71 There are no other associated neurologic abnormalities; the CSF may show oligoclonal bands and increased IgG index, and neuroradiologic studies are usually normal.72 The tumors most commonly involved include breast cancer, SCLC, thymoma, and Hodgkin disease. A subset of patients with paraneoplastic stiff man syndrome and breast cancer or SCLC have antibodies that react with amphiphysin, a neuronal synaptic protein.71,73,74 When stiff man syndrome occurs in patients who do not have cancer, the disorder is associated with antibodies against glutamic acid decarboxylase.77–77