Opioids
Perspective
Opioid is a term that applies to all natural, synthetic, and semisynthetic agents with morphine-like actions. It is more inclusive than the term opiate, which refers only to natural agents. Both terms are derived from opium, the Greek word for juice in reference to poppy juice. Poppy juice contains more than 20 distinct natural alkaloids with morphine-like activity. The nonspecific term narcotic refers to any agent that induces sleep and should not be used. Opioid is more precise and is the correct medical term for the agents that act on opiate receptors in the body. Finally, the term endorphin applies to any of the endogenous opioid peptides, including endomorphins, dynorphins, enkephalins, and nociceptin.1
The World Drug Report 2010 of the United Nations Office on Drugs and Crime estimates that between 155 and 250 million people (3.5 to 5.7% of the population aged 15 to 64 years) used illicit substances at least once in 2008. Globally, cannabis, amphetamine-type stimulant, and cocaine users outnumber opiate (heroin/opium) users, but opiates are associated with more harm with use. Annual prevalence of opiate (heroin/opium) use in the United States is estimated at 0.58% of the population aged 15 to 64 years. Mortality rate for dependent opiate users is between 6 and 20 times that expected for those in the general population of the same age and gender.2
In addition, nonmedicinal use of prescription opioids, in adults and teens, is an extensive and growing problem in the United States. According to the Centers for Disease Control and Prevention (CDC), in 2010, enough opioid analgesics were sold to medicate every American adult with a typical dose of 5 mg of hydrocodone every 4 hours for 1 month.3 Fatal poisonings involving opioid analgesics more than tripled from 4000 to 13,800 from 1999 to 2006, including a multistate epidemic of nonpharmaceutical fentanyl-related overdoses that resulted in more than 1000 deaths from April 2006 to March 2007.4 This trend continues to rise, and in 2010 there were more than 15,000 opioid analgesic–related fatalities reported to the CDC.
Principles of Disease
Although opioids have been used for more than 5000 years, opiate binding to brain membrane receptors was not shown until the 1970s, and the first opioid receptor gene was not isolated until 1992. Subsequent molecular characterization of the endogenous opioid system, including peptides and receptors, has improved the understanding of opioid physiologic roles.5
The three established opioid receptors, mu/MOP (OPRM1 gene), kappa/KOP (OPRK1 gene), and delta/DOP (OPRD1 gene), are transmembrane, G protein–coupled receptors distributed throughout the CNS, concentrated in areas associated with nociception as well as in areas of euphoria and respiratory control. Systemically, opioid receptors are localized in sensory nerve endings, the gastrointestinal tract, cardiovascular endothelial cells, and immune cells.5,6 Whereas opioid receptor ligand binding has demonstrated variable pharmacologic effects, whether it is through specific opioid receptor subtypes or receptor gene splice variants, receptor dimerization, or biased ligand agonism is unclear.7 Genetic variations in opioid receptors and in metabolic pathways account for some of the interindividual differences in response to both endogenous and exogenous opioids. Several polymorphisms of OPRM1 have been identified, including A118G polymorphism, present in up to 30% of individuals of European descent and 50% of Asian descent,8 which is associated with increased dosage requirements for pain control. Whereas it might also result in lower relative clinical toxicity after overdose, this has not been studied. Polymorphisms of several cytochrome P450 enzymes, including CYP2D6 (tramadol, codeine), CYP3A5 (fentanyl), and CYP2B6 (methadone), have been linked to variability in response to several opioids.9
Pathophysiology and Pharmacology
Opioids are well absorbed after gastrointestinal (oral and rectal) or parenteral administration but also through nasal, buccal, pulmonary, and transdermal routes, depending on the lipid solubility of the specific opioid.1 Heroin is usually abused through intravenous and subcutaneous routes, but it is also absorbed after nasal administration because it is lipid soluble. In general, opioid toxicity is less pronounced but more prolonged with ingestion than with parenteral administration.1 Absorption of opioids after ingestion occurs in the small intestine and with therapeutic doses is complete within 1 or 2 hours. However, because of delayed gastric emptying, absorption and clinical effects of toxicity may be prolonged after overdose.
Most opioids have a large volume of distribution. Clinical effects depend on lipid solubility, which affects the rate at which opioids and their metabolites cross the blood-brain barrier. All opioids undergo hepatic metabolism and renal elimination, and variations in hepatic and renal function are important because metabolite activity may contribute to clinical effects and toxicity.1
The pharmacokinetics of the specific agent determine the clinical course of opioid toxicity. Heroin peaks in the serum within 1 minute of intravenous injection, 3 to 5 minutes of intranasal administration, and 10 minutes of subcutaneous injection. Heroin’s lipophilic nature allows rapid transport across the blood-brain barrier into the CNS. In the CNS and blood, heroin is rapidly hydrolyzed to 6-monoacetylmorphine and then morphine, which is less lipid soluble. In the liver, morphine undergoes conjugation with glucuronic acid to form more water-soluble compounds that are excreted by the kidneys.1 Morphine and heroin are not metabolized by cytochrome P450 enzymes and are not directly affected by P450 polymorphisms.
Withdrawal
Because the half-life of heroin is 30 minutes and the half-life of methadone is 15 to 40 hours, withdrawal symptoms occur 4 to 6 hours after discontinuation of heroin and 24 to 48 hours after discontinuation of methadone.10,11 Duration of symptoms is 7 to 10 days and 2 weeks, respectively. The degree of physical dependence that has developed is also important. With chronic opioid exposure, cellular adaptation results in upregulation of cyclic adenosine monophosphate (cAMP). When the exposure is discontinued or an antagonist is administered, the result is a temporary elevation of cAMP levels and increased sympathetic activity above a normal baseline.
Clinical Features
Neurologic
Parkinsonian symptoms in injection drug abusers have been attributed to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), a side product during synthesis of a meperidine analogue in street laboratories. MPTP metabolite accumulates in CNS cell mitochondria and is associated with focal lesions in the substantia nigra and a syndrome clinically indistinguishable from idiopathic parkinsonism. The syndrome is irreversible in some patients.12
Spongiform leukoencephalopathy is associated with “chasing the dragon,” a practice of inhaling heroin heated typically on aluminum foil. Patients have psychomotor retardation, dysarthria, ataxia, tremor, and other neurologic abnormalities.13 This syndrome is thought to be related to a combination of mitochondrial injury and hypoxia from the heroin or, more likely, the method of preparation. One report also associates heroin-induced movement disturbance with basal ganglia lesions.14
Serotonin syndrome is a clinical triad of mental status changes, autonomic instability, and neuromuscular changes (see Chapter 151) and may be fatal. Most cases involve an interaction between a serotonergic agent and a second agent, usually a selective serotonin reuptake inhibitor or a monoamine oxidase inhibitor. Meperidine, dextromethorphan, methadone, and tramadol inhibit serotonin reuptake and are associated with serotonin syndrome, as are some opiate analogues without known serotonin reuptake effects, such as oxycodone and buprenorphine.15
Respiratory
By suppressing the sensitivity of the medullary respiratory center to hypercapnia, opioids decrease respiratory rate and tidal volume in a dose-dependent manner.1 Although it initially remains intact, the hypoxic drive is overridden in severe poisoning or when antagonistic stimuli (e.g., pain) are blocked. Overdose of an agonist-antagonist agent produces less significant respiratory depression, presumably because of mu receptor antagonism (Table 162-1). Central sleep apnea occurs with long-term opioid use and also with acute increased opioid use from baseline. Continuous positive airway pressure is usually ineffective for treatment of sleep apnea in these patients.16
Table 162-1
Select Opioid Doses and Associated Respiratory Depression
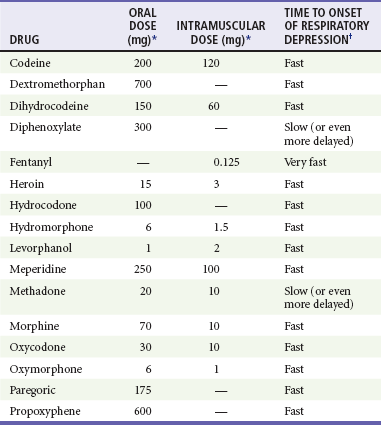
*Equivalent to 10 mg of intramuscular morphine.
†Varies with the drug and route of administration. In addition, the effects of a dose in any particular patient depend on multiple factors, including age, weight, and comorbid conditions. After intramuscular administration, very fast means 5 to 30 minutes, fast means 15 to 60 minutes, and slow means 1 to 4 hours. After oral administration, these time definitions are approximately doubled.
Bronchospasm is rare with heroin use in both asthmatic and nonasthmatic patients after inhalational exposure and rarely after other routes. When it occurs, the bronchospasm is often severe, prolonged, and refractory to beta-agonist therapy, and it may require mechanical ventilation for several days. It is unclear whether the heroin, an adulterant, or a combination triggers the bronchospasm and whether the response is histamine mediated or from direct irritation.17
Acute lung injury occurs with therapeutic opioid use but is much more common after overdose.18 The capillary leak is likely to be from hypoxia rather than a direct drug effect.



