Normally Sterile Body Fluids, Bone and Bone Marrow, and Solid Tissues
1. Describe the fine main cavities of the human body; also name the membranes associated with these cavities and state the function of these membranes.
2. Define each of the following body cavity fluids, and explain the diagnostic culture methods for each: pleural fluid, pericardial fluid, peritoneal fluid, joint fluid, and dialysis fluid.
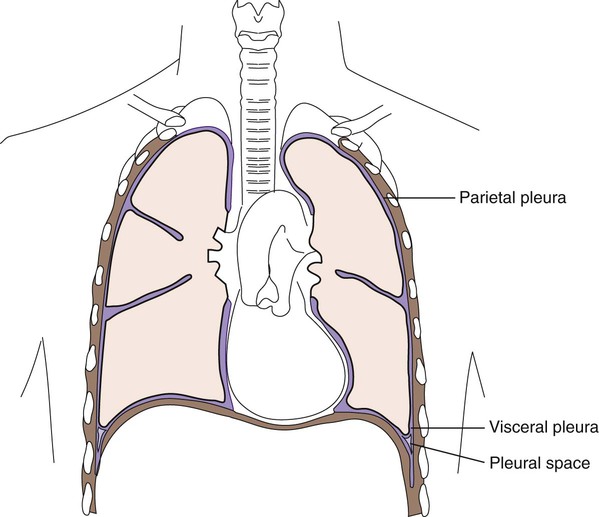
3. Define parietal and visceral pleura.
4. Define cellulitis; name the etiologic agents of this illness, and explain the associated risk factors for the development of disease.
5. Define pleural effusion; explain the difference between exudative pleural effusion and transudative pleural effusion.
6. Explain when a pleural effusion becomes an empyema and what medical condition contributes to the development of an empyema?
7. Define pericarditis and myocarditis; explain the physical conditions that may contribute to the accumulation of pericardial fluid.
8. Define peritonitis and differentiate between primary and secondary peritonitis.
9. Name the etiologic agents most commonly isolated from primary peritonitis cases in children, adults, sexually active females, and immunocompromised patients.
10. Define osteomyelitis; explain how this infection is transmitted, the diagnostic method, and the organisms most frequently responsible for this type of infection.
11. Explain the process for culturing organisms from the following specimens: bone, tissue, and bone marrow.
12. Correlate patient signs and symptoms with laboratory results to identify the etiologic agent associated with the body fluid, bone and bone marrow, and other solid tissue infection.
Bacteria, fungi, virus, or parasite can invade any body tissue or sterile body fluid site. Although from different areas of the body, all specimens discussed in this chapter are considered normally sterile. Therefore, even one colony of a potentially pathogenic microorganism may be significant. (Refer to Table 5-1 for a quick guide regarding collection, transport, and processing of specimens from sterile body sites.)
Specimens From Sterile Body Sites
Fluids
In response to infection, fluid may accumulate in any body cavity. Infected solid tissue often presents as cellulitis or with abscess formation. Areas of the body from which fluids are typically sent for microbiologic studies (in addition to blood and cerebrospinal fluid [see Chapters 68 and 71]) include those in Table 77-1.
TABLE 77-1
Microbiology Laboratory Body Fluid Collection Sites
| Body Area | Fluid Name(s) |
| Thorax | Thoracentesis or pleural or empyema fluid |
| Abdominal cavity | Paracentesis or ascitic or peritoneal fluid |
| Joint | Synovial fluid |
| Pericardium | Pericardial fluid |
Pleural Fluid
Lining the entire thoracic cavity (see Chapter 69) of the body is a serous membrane called the parietal pleura. Covering the outer surface of the lung is another membrane called the visceral pleura (Figure 77-1). Within the pleural space between the lung and chest wall is a small amount of fluid called pleural fluid that lubricates the surfaces of the pleura (the membranes surrounding the lungs and lining the chest cavity). Normally, equilibrium exists among the pleural membranes, but in certain disease states, such as cardiac, hepatic, or renal disease, excess amounts of this fluid can be produced and accumulates in the pleural space; this is known as a pleural effusion. Pleural effusions can either be exudative or transudative. Exudative pleural effusions are caused by inflammation, infection, and cancer, whereas transudative effusions are due to systemic changes, such as congestive heart failure.
Normal pleural fluid contains few or no cells and has a consistency similar to serum, but with a lower protein count. Pleural fluid containing numerous white blood cells is indicative of infections. Pleural fluid specimens are collected by thoracentesis, a procedure in which a needle is inserted through the chest wall into the pleural space and the excess fluid aspirated. This fluid is then submitted to the laboratory as thoracentesis fluid, pleural fluid, or empyema fluid. The fluid, or effusion, can then be analyzed for cell count, total protein, glucose, lactate dehydrogenase, amylase, cytology, and culture. The total protein and glucose results determine if the effusion is transudate or exudate. The patient’s serum or plasma glucose level is needed to compare with the results indicated in the body fluid. Several characteristics can be used to determine whether a fluid is a transudate or exudate (Table 77-2). When effusions are extremely purulent or full of pus, the effusion is referred to as an empyema. Empyema often arises as a complication of pneumonia, but other infections near the lung (e.g., subdiaphragmatic infection) may seed microorganisms into the pleural cavity. It has been estimated that 50% to 60% of patients develop empyema as a complication of pneumonia.
TABLE 77-2
Pleural Fluid Effusion Characteristics
| Transudate | Exudate | |
| Appearance | Clear | Cloudy |
| Specific Gravity | <1.015 | >1.015 |
| Total Protein | <3.0 mg/dL | >3.0 mg/dL |
| LD Fluid: Serum Ratio | <0.6 | >0.6 |
| Cholesterol | <60 mg/dL | >60 mg/dL |
| Cholesterol Fluid: Serum Ratio | <0.3 | >0.3 |
| Bilirubin Fluid:Serum Ratio | <0.6 | >0.6 |
| Total Protein Fluid: Serum Ratio | <0.5 | >0.6 |
| White Blood Cells | <1000/µL (all white blood cell types, all <50%) | >1000/µL |
| Red Blood Cells | <10,000/µL = because of traumatic tap | >100,000/µL |
| Clotting | Will not clot | May clot |
Modified from Strasinger SK, Di Lorenzo MS: Urinalysis and body fluids, ed 5, Philadelphia, 2008, F.A. Davis.
Peritoneal Fluid
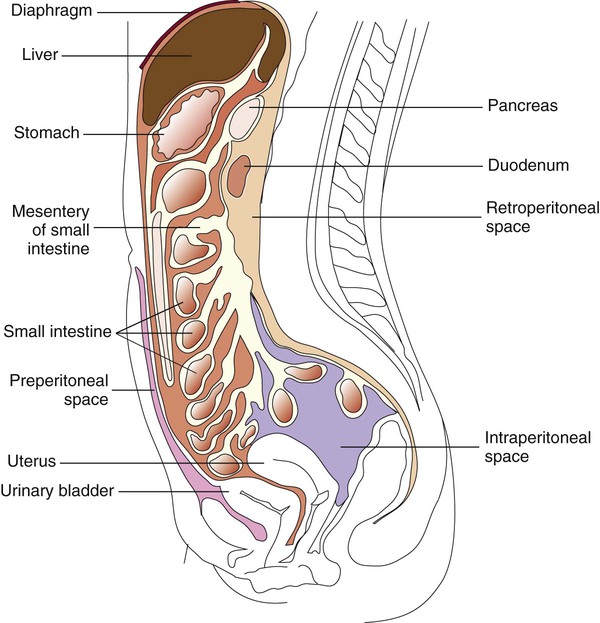
The peritoneum is a large, moist, continuous sheet of serous membrane lining the walls of the abdominal-pelvic cavity and the outer coat of the organs contained within the cavity (Figure 77-2). In the abdomen, these two membrane linings are separated by a space called the peritoneal cavity, which contains or abuts the liver, pancreas, spleen, stomach and intestinal tract, bladder, and fallopian tubes and ovaries. The kidneys occupy a retroperitoneal (behind the peritoneum) position. Within the healthy human peritoneal cavity is a small amount of fluid that maintains the surface moisture of the peritoneum. Normal peritoneal fluid may contain as many as 300 white blood cells per milliliter, but the protein content and specific gravity of the fluid are low. During an infectious or inflammatory process, increased amounts of fluid accumulate in the peritoneal cavity, a condition called ascites. Most cases of ascites are due to liver disease, and in severe cases, the abdomen is often distended. The fluid can be collected for testing by paracentesis (the insertion of a needle into the abdomen and removal of fluid). The peritoneal or ascites fluid can then be analyzed for amylase, protein, albumin, cell count, culture, and cytology. Often ascitic fluid contains an increased number of inflammatory cells and an elevated protein level.
Pericardial Fluid
Myocarditis (inflammation of the heart muscle itself) may accompany or follow pericarditis. The pathogenesis of disease involves the host inflammatory response contributing to fluid buildup as well as cell and tissue damage. Common causes of myocarditis include viral infections with Coxsackie virus, echoviruses, or adenovirus. The most common etiologic agents of pericarditis and myocarditis are listed in Box 77-1. Other bacteria, fungi, and parasitic agents have been recovered from pericardial effusions.
Joint Fluid
Overall, Staphylococcus aureus is the most common etiologic agent of septic arthritis, accounting for approximately 70% of infections. In adults younger than 30 years of age, however, Neisseria gonorrhoeae is isolated most frequently. Haemophilus influenzae has been the most common agent of bacteremia in children younger than 2 years of age, and consequently it has been the most frequent cause of infectious arthritis in these patients, followed by S. aureus. The widespread use of H. influenzae type B vaccine should contribute to a change in this pattern. Streptococci, including groups A (Streptococcus pyogenes) and B (Streptococcus agalactiae), pneumococci, and viridans streptococci, are prominent among bacterial agents associated with infectious arthritis in patients of all ages. Among anaerobic bacteria, Bacteroides, including B. fragilis, may be recovered and Fusobacterium necrophorum, which usually involves more than one joint in the course of sepsis. Among people living in certain endemic areas of the United States and Europe, infectious arthritis is a prominent feature associated with Lyme disease. Chronic monoarticular arthritis is frequently due to mycobacteria, Nocardia asteroides, and fungi. Some of the more frequently encountered etiologic agents of infectious arthritis are listed in Box 77-2.