Chapter 31 Management of Tumors of the Fourth Ventricle
Anatomy
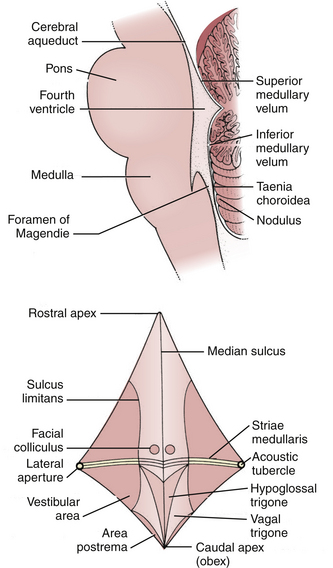
The fourth ventricle is a broad tent-shaped cerebrospinal fluid (CSF) cavity located behind the brain stem and in front of the cerebellum in the center of the posterior fossa (Fig. 31-1). CSF enters through the cerebral aqueduct, which opens into the fourth ventricle at its rostral end. The ventricle widens caudally until its maximum width at the level of the lateral recesses, from which CSF exits through the two foramina of Luschka into the cerebellopontine cisterns on either side. The ventricle narrows again to its caudal terminus at the obliterated central canal of the spinal cord, called the obex from the Latin for “barrier.” The foramen of Magendie is just posterior to the obex and allows CSF to exit into the cerebellomedullary cistern, which is continuous with the cisterna magna. There are no arteries or veins within the cavity of the fourth ventricle. All of the vessels associated with this region are in the fissures located just outside the fourth ventricular roof.
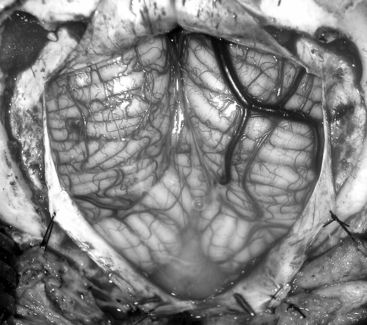
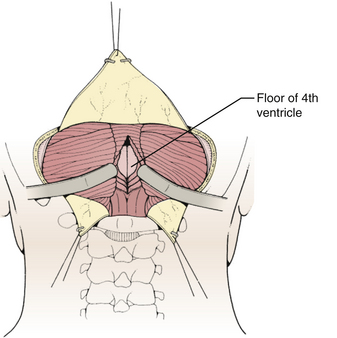
The glistening white floor of the fourth ventricle is the posterior surface of the brain stem (Fig. 31-2). The border between the pons and medulla occurs approximately at the level of the foramina of Luschka. The superior (pontine) part of the floor begins at the aqueduct and expands to the lower margin of the cerebellar peduncles. The inferior (medullary) part of the floor begins just below the lateral recesses at the attachment of the tela choroidea to the taenia choroidea and extends to the obex, limited laterally be the taeniae, which mark the inferolateral margins of the floor. Between these is the intermediate part, which extends into the lateral recesses on either side. There is a longitudinal midline sulcus in the fourth ventricular floor called the median sulcus. On either side of the median sulcus is the sulcus limitans, which also runs longitudinally parallel to the median sulcus. The sulcus limitans is an important landmark for functional anatomy of nuclei beneath the ventricular floor, as motor nuclei are medial and sensory nuclei lateral to the sulcus limitans. Medial to the sulcus limitans on either side of the median sulcus is the median eminence, a collection of four paired elevations in the fourth ventricular floor that are collectively referred to as the calamus scriptorius since they resemble the head of a fountain pen (Fig. 31-1). Rostral to caudal, the median eminence consists of the facial colliculus, which overlies the facial nucleus; the hypoglossal triangle, which overlies the hypoglossal nucleus; the vagal triangle, which overlies the dorsal nucleus of the vagus; and the area postrema, a tongue-shaped structure that is part of the brain-stem emetic center. Lateral to the sulcus limitans is the vestibular area, so named because is overlies the vestibular nuclei. This area is widest in the neighborhood of the lateral recess, where the striae medullaris cross transversely across the inferior cerebellar peduncles to disappear into the median sulcus. The auditory tubercle in the lateral part of the vestibular area overlies the dorsal cochlear nucleus and cochlear nerve.
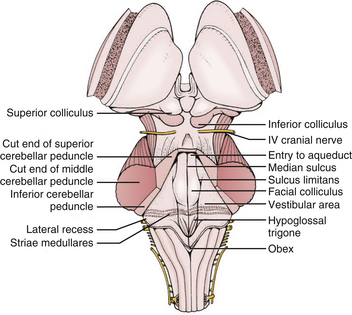
FIGURE 31-2 Fourth ventricle after removal of the cerebellum.
(Modified from Cohen AR. Surgical Disorders of the Fourth Ventricle. Cambridge, MA: Blackwell Science; 1996.)
The roof of the fourth ventricle is tent-shaped, rising to an apex called the fastigium that divides the superior roof from the inferior roof. The median part of the superior roof, called the superior medullary velum, consists of a thin lamina of white matter between the cerebellar peduncles. Just behind its outer surface is the lingula, the uppermost division of the vermis. The lateral walls of the superior roof are formed by the superior and inferior cerebellar peduncles, which lie between the fourth ventricle and the middle cerebellar peduncle. The rostral midline of the inferior roof is formed by the nodule, which lies directly in front of the uvula, the lower part of the vermis that hangs down between the tonsils (mimicking the appearance of the pharynx). Lateral to the nodule is the inferior medullary velum, a thin sheet of neural tissue that stretches over the fourth ventricle to connect the nodule to the flocculi on either side just superior to the outer extremity of the lateral recess. The inferior medullary velum is thus part of the primitive flocculonodular lobe of the cerebellum. The caudal inferior roof consists of the tela choroidea, two thin arachnoid-like membranes sandwiching a vascular layer of choroidal vessels to which the choroid plexus is attached. The junction between the tela choroidea and the nodule/inferior medullary velum (telovelar junction) is at the level of the lateral recess. The tela choroidea is attached to the ventricular floor at narrow white ridges called taeniae choroidea, which meet at the obex and extend upward to turn laterally over the inferior cerebellar peduncles into each lateral recess, forming its lower border. As a result, the choroid plexus (extending from the ventricular surface of the tela) forms an upside-down L shape on either side of midline. There is a medial segment of choroid plexus that extends longitudinally from the foramen of Magendie up to the nodule and a lateral segment that extends transversely from the rostral ends of the medial segments out to the foramen of Luschka. The three fourth ventricular outlet foramina (Magendie and Luschka) are located in the tela choroidea itself, and frequently choroid plexus protrudes from these foramina.
The cerebellopontine fissures are intimately related to the lateral recesses of the fourth ventricle. They are produced by the folding of the cerebellum laterally around the sides of the pons and middle cerebellar peduncles. Each cerebellopontine fissure is shaped like a V in the coronal plain with the point facing laterally. The outer surface of the V is made up of the petrosal surfaces of the cerebellum, and the inner surface is made up of the middle cerebellar peduncles. The lateral recess and foramen of Luschka open into the medial part of the inferior limb of the V near the flocculus. Several cranial nerves run through the cerebellopontine fissure, including the trigeminal (through the superior limb) and the facial, glossopharyngeal, and vagus (through the inferior limb). The anterior inferior cerebellar arteries (AICA) also run through these fissures. Each AICA courses posteriorly around the pons then sends branches to nerves of the acoustic meatus and choroid plexus protruding from the foramen of Luschka before passing around the flocculus on the middle cerebellar peduncle to supply the petrosal surface of the cerebellum. Venous blood from the cerebellopontine fissure and lateral recess primarily drains into the superior petrosal sinus. The vein of the cerebellopontine fissure is formed by the convergence of several veins on the apex of the fissure, including the vein of the middle cerebellar peduncle into which the vein of the inferior cerebellar peduncle drains. This vein courses near the superior limb of the fissure to drain into the superior petrosal sinus rostral to the facial and glossopharyngeal nerves.
Surgical Technique
Surgical Approach
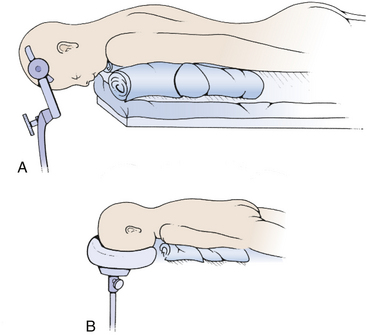
The most commonly used position for the midline suboccipital approach (especially in very young patients) is the prone position, in which the patient is rolled after induction of anesthesia so that the face is toward the floor (Fig. 31-3). There are many advantages to this position: the anatomy is clearly visualized, it is easy for two to work together since one operator can stand on either side, and the multiple complications of the sitting position do not occur. The most significant disadvantage of the prone position is venous congestion that can lead to more significant blood loss, pooling of blood in the operative field, and soft tissue swelling of the face. This congestion is much worse if the head is rotated and flexed, and is improved somewhat by elevating the head above the level of the heart. Also, nasotracheal rather than orotracheal intubation can minimize compression of the base of tongue and impairment of venous drainage of the tongue and pharynx. The weight is distributed to minimize pressure points that can lead to skin breakdown and neuropathy, especially at the ulnar nerve at the elbow, common peroneal nerve across fibular head, and lateral femoral cutaneous nerve at the iliac crest. Two longitudinal padded roles are placed under the patient, and the knees and ankles are padded. The neck is placed in the “military tuck position” with moderate flexion of the upper cervical spine (to open up the space between the foramen magnum and the arch of C1) and less flexion of the lower cervical spine (to bring the occiput parallel with the patient’s back). The chin and chest at least two fingers apart. Finally, the table is positioned so that the neck is parallel to floor and the head is above the heart. The shoulders can be gently retracted toward the feet with some tape, and a strap under the buttocks is helpful to prevent sliding. The surgeon and assistant then operate from either side using the microscope, and the scrub nurse’s Mayfield table can be placed over the patient’s back.
The third option for positioning is the sitting position, in which the patient is positioned sitting upright so that the operative corridor is parallel to the floor (Fig. 31-4). The sitting position offers a very clear operative field since blood and cerebrospinal fluid drain out of the operative site. However, there are many risks to the sitting position.1 The most significant dangers are cardiovascular instability and hypotension, air embolism, and subdural hematoma. All patients should have an agitated saline echocardiogram to exclude right to left shunt through a patent foramen ovale that could complicate air embolism and presence of such a shunt is an absolute contraindication for the sitting position. Precordial Doppler ultrasonic flow and end-tidal CO2 should be monitored throughout the case. The risk of subdural hematoma is greatly increased by presence of a shunt, and if possible the shunt should be occluded prior to attempting an operation in the sitting position. Other risks of the sitting position include tension pneumocephalus, cervical myelopathy, thermal loss (especially in children), surgeon fatigue, and sudden loss of CSF from enlarged lateral and third ventricles after removal of a fourth ventricle mass lesion. When applying the head holder, the pin sites must be covered with Vaseline gauze to minimize entry of air2 and the head taped to the head-holder for extra support in case the pins become dislodged. The patient is elevated slowly into the sitting position so that the foramen magnum is at the surgeon’s eye level with both of the patient’s legs flexed at the knees to prevent postoperative sciatica. The instrument table is placed over the patient’s head. Infants too young for pins may be taped to a padded headrest to support the forehead and chin, but it is probably safer to use the prone position. Throughout the case the patient should be carefully monitored for signs of hypotension or air embolism. If air embolism occurs, the wound should be packed with a saline-soaked sponge, and anesthesia should aspirate the atrial catheter to attempt to remove the embolus from the left atrium. If the embolus is severe, the patient should be placed in left decubitus position; otherwise, as soon as the patient is stable, the wound may be slowly exposed while covering the potential source of air with Gelfoam and Surgicel. If careful preparation is undertaken and complications dealt with promptly, the sitting position can be relatively safe.3,4
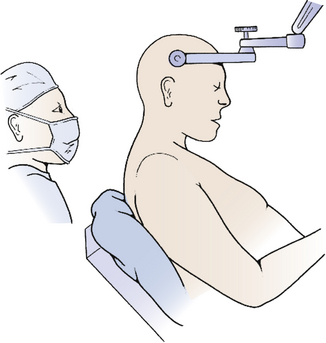
FIGURE 31-4 Sitting position. Skeletal fixation is maintained using a pin head holder with the neck moderately flexed.
(Modified from Cohen AR. Surgical Disorders of the Fourth Ventricle. Cambridge, MA: Blackwell Science; 1996.)
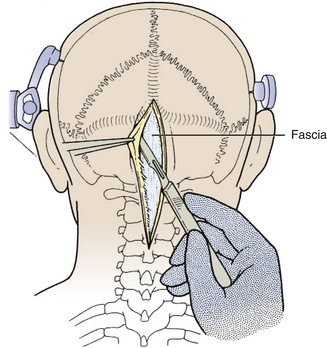
The incision is made with a number 10 blade applying firm digital compression, and bleeding points are coagulated (Fig. 31-5). The incision should be midline, but if the tumor is lateral, a hockey-stick incision can be used to allow for a wider craniectomy. The skin is undermined superficial to the fascia on both sides of the superior half of incision in preparation to create a fascial flap for closure (Fig. 31-6). The skin is then elevated with toothed forceps or a skin hook and a plane of dissection developed with knife or monopolar coagulation, sparing the occipital artery and nerve whenever possible. Even a slight deviation off midline will produce brisk bleeding from the muscles once deeper tissues are exposed. When anatomical landmarks are identified to confirm that the operative course is truly midline, cerebellar or Weitlaner retractors are placed to maintain exposure. As deeper layers are exposed, curved retractors may be used.
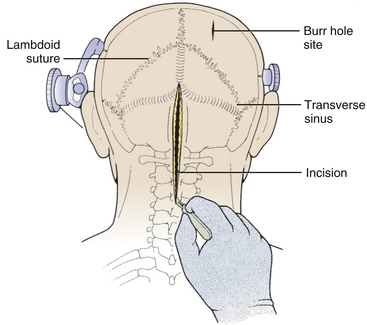
FIGURE 31-5 Skin Incision. The midline linear incision extends from just above the inion to the midcervical region.
(Modified from Cohen AR. Surgical Disorders of the Fourth Ventricle. Cambridge, MA: Blackwell Science; 1996.)
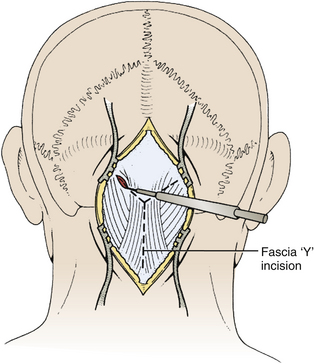
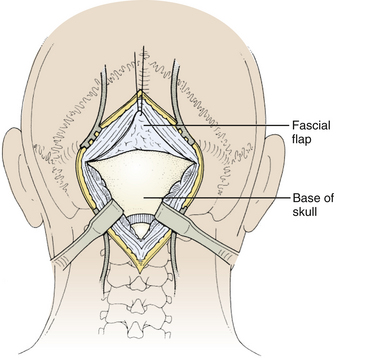
Next, the fascia is incised using a Y-shaped incision, keeping the lateral ends of the Y below the ligamentous insertion (Fig. 31-7). While a linear midline fascial incision without the upper limbs of the Y allows use of the avascular plane between the splenius capitus and semispinalis capitis muscles, it is often difficult to reapproximate such an incision tightly at the superior nuchal line. Muscle flaps are then developed with monopolar cautery and periosteal elevators, stripping the muscle from the bone as far as the mastoid emissary vein. This exposure is maintained with two curved cerebellar retractors and the rostral flap is placed under tension using a 3-0 silk suture to reflect it rostrally (Fig. 31-8). The muscle insertions are stripped off the spinous process and laminae of C2. Finally, the junction between the pericranium and dura at the foramen magnum is sharply dissected, and then the posterior fossa dura separated from the inner table of the occipital bone using a curette.
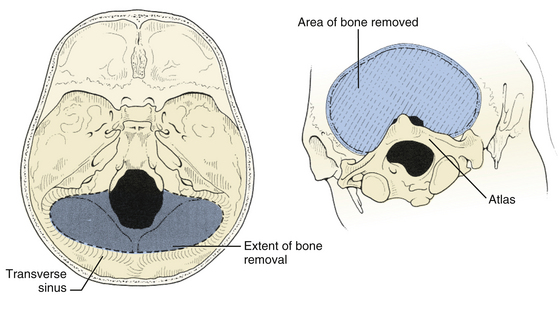
The suboccipital craniotomy is begun with burr holes on either side of midline just below the transverse sinuses, about three centimeters from midline (Fig. 31-9). A third burr hole can be placed below the torcular in older patients. In children, the dura is not firmly adherent to the skull so it is safe to drill close to or even on top of the sinuses, but more caution must be used with adults. The dura near the burr hole is then stripped using a Penfield and the bone removed using a high speed. The superior and lateral limits of the craniotomy are the transverse and sigmoid sinuses (Fig. 31-10). Inferiorly, the craniotomy should always include the posterior edge of the foramen magnum to prevent laceration of the brain against the closed bony rim when cerebellar elements are retracted downward and minimize damage from herniation if hematoma or swelling should occur postoperatively. The midline bone is removed last since it is often very vascular and contains a keel that can be quite deep. This keel must be stripped of dura with a Penfield, using extreme caution near the occipital sinus in the midline and the annular sinus near the foramen magnum. All exposed bone edges should be waxed, especially in the sitting position. Because of the irregular contour of the inner bone surface in adult patients, it is sometimes necessary perform a craniectomy rather than a craniotomy, removing the bone in a piecemeal fashion.
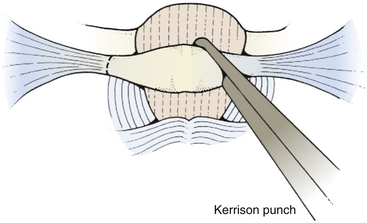
To expose the posterior arch of C1, the soft tissues overlying it are reflected laterally using a small periosteal elevator, stripping the inferior arch first since the vertebral artery is on its superior aspect. It is sometimes easier to do this after C2 has been exposed. The periosteum can sometimes be swept off the arch of C1 using an index finger covered with gauze. Monopolar cautery should be used with caution when dissecting the soft tissue over C1 (especially at the superolateral surface) to prevent injury to vertebral artery. It is important to remember that C1 can be bifid and is often cartilaginous in infants and young children. C1 laminectomy is helpful for lesions that herniate beneath the foramen magnum. To remove the lamina, small angled curettes can be used to strip the deep surface of the bone, and then the bone itself removed with an angled Kerrison punch or Leksell rongeur (Fig. 31-11). Because extending a laminectomy below C2 in young children increases the risk of swan neck deformity,5 it is prudent to remove the smallest amount of bone possible. For most tumors, it is usually only necessary to remove as far as one level above the most caudal aspect of the tumor.
Prior to the dural incision, the wound should be irrigated and retractor systems and microscope prepared (Fig. 31-12). If the dura is tense, the intracranial pressure can be reduced with external ventricular drainage (if available), hyperventilation, or mannitol, although mannitol should be used with caution in the sitting position as it has been implicated in the development of subdural hematomas. All techniques for dural incision require crossing the occipital and annular sinuses, which may be very large in infants under age 2 years and can persist until 25 years of age. A Y-shaped incision allows wide visualization and can be extended if necessary (Fig. 31-13). One superior limb should be incised first with a number-15 blade. The incision should start just inferior to the transverse sinus and travel obliquely to the midline, stopping short of the occipital sinus. The other superior limb is incised next, and then they are connected over the midline. If there is significant bleeding from the midline occipital sinus, it should be controlled with obliquely placed hemostatic clips or suture ligatures (Fig. 31-14). Either way, both the superficial and deep layer of the dura must be incised or the sinus will be tented open. The vertical limb of the Y is opened last using scissors so that the dura can be tented if bleeding is seen. The vertical incision extends to the foramen magnum so that it will extend below the falx cerebelli, which is occasionally present in childhood. If bleeding is very troublesome, the dura can be opened paramidline. The dura is then covered with a moist collagen sponge or wet Gelfoam sandwich to prevent desiccation and anchored to the fascia with 4-0 neurolon suture. This allows wide exposure of the cerebellar vermis and hemispheres (Figs. 31-15 and 31-16). The arachnoid is opened next over the cisterna magna to allow drainage of CSF (Fig. 31-17). If the tumor is in the cerebellar hemisphere, another dural incision can be extended laterally to more fully expose the involved cerebellum.
FIGURE 31-12 Dural exposure after removal of bone flap. The arch of C1 has been preserved in this patient.
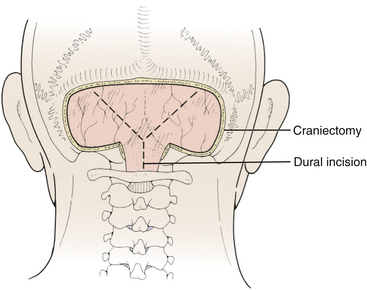
FIGURE 31-13 Dural incision. The vertical limb of the Y-shaped incision, which overlies the occipital sinus, is opened last.
(Modified from Cohen AR. Surgical Disorders of the Fourth Ventricle. Cambridge, MA: Blackwell Science; 1996.)
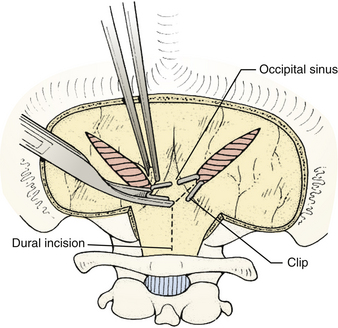
FIGURE 31-14 Control of bleeding from occipital sinus with clips.
(Modified from Cohen AR. Surgical Disorders of the Fourth Ventricle. Cambridge, MA: Blackwell Science; 1996.)
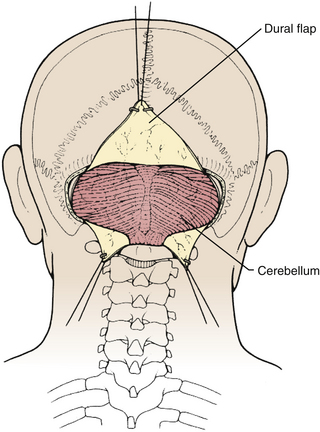
FIGURE 31-15 Exposure of cerebellar hemispheres and vermis.
(Modified from Cohen AR. Surgical Disorders of the Fourth Ventricle. Cambridge, MA: Blackwell Science; 1996.)
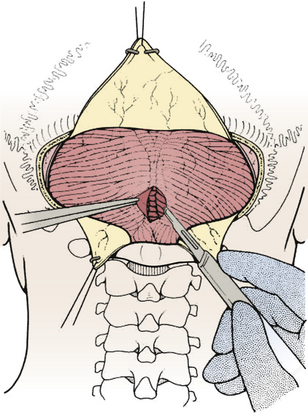
FIGURE 31-17 Opening the cisterna magna. This allows drainage of CSF and relaxes the posterior fossa.
(Modified from Cohen AR. Surgical Disorders of the Fourth Ventricle. Cambridge, MA: Blackwell Science; 1996.)
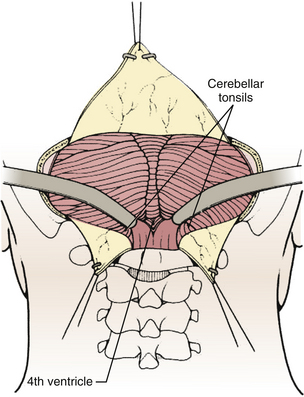
Techniques for intradural exposure and resection of the tumor will vary depending upon the location and size of the tumor, and will be discussed in more detail for each individual tumor. Gentle separation of the cerebellar tonsils will expose the cerebellomedullary fissure through the opened vallecula giving an unimpeded view of the inferior roof of the fourth ventricle (Fig. 31-18). Narrow malleable automatic retractors can be used to maintain separation of the tonsils; the retractor system should be kept close to the patient so as not to interfere with the subsequent operation. The operating microscope is brought into the field and the anatomy is identified. In particular, the location of the caudal loops of PICA should be carefully noted since they are often tethered to the tonsils and the walls of the cerebellomedullary fissure by small perforating branches. The foramen of Magendie and the small tuft of choroid plexus protruding from it will be clearly seen, as well as any tumor that protrudes from the foramen. The thin layers forming the lower part of the roof can be opened to expose the cavity of the fourth ventricle. Often this will provide sufficient exposure, but if not, it is sometimes helpful to retract the inferior vermis rostrally or incise the caudal vermis, avoiding the gutter between the vermis and the hemisphere to prevent injury to the inferior vermian veins there (Fig. 31-19). Lateral lesions may require removal of one tonsil by dividing the pedicle attaching the superolateral margin of the tonsil to the biventral lobule. To reach the lateral roof or lateral recess, part of the cerebellar hemisphere can be resected without significant morbidity as long as the dentate nuclei are not violated. If the tumor is not adherent to the floor of the fourth ventricle, cottonoid patties should be placed beneath the tumor to protect the delicate brain-stem structures just beneath the floor. These cottonoids should be placed under direct vision and never used as a tool to dissect the tumor from the floor of the fourth ventricle. After the tumor has been removed, the glistening white floor of the fourth ventricle should be clearly visible. The retractors are then removed and the cerebellar hemispheres allowed to fall back into place. If there is extension of the tumor through one of the foramina of Luschka into the cerebellopontine cistern, the ipsilateral tonsil and cerebellar hemisphere can be retracted medially to expose it. Sometimes it is necessary to do a secondary retromastoid approach to completely resect the tumor.
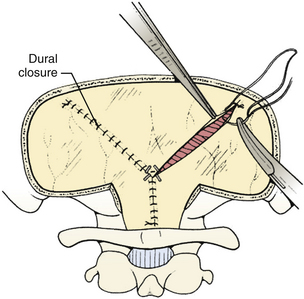
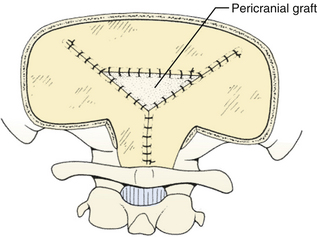
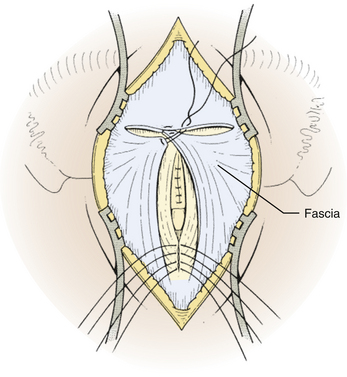
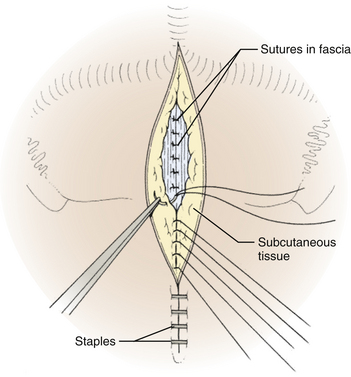
The dura is closed using a running 4-0 neurolon or polypropylene after approximating the dural edges with interrupted sutures (Fig. 31-20). A Valsalva maneuver will identify potentially dangerous venous bleeding. The dural closure should be watertight if possible, starting peripherally then working centrally to gradually overcome the tension. If the dura is not watertight, there is increased risk of pseudomeningocele due to a ball-valve effect or hydrocephalus from arachnoid adhesions produced by blood from the muscles. Sometimes the dura will be dried and shrunken by the end of the case, especially if measures have been taken to obliterate the occipital sinus. In this case, the remaining defect can be covered with a pericranial or fascial graft (Fig. 31-21). Freeze-dried bovine pericardium or human allograft dura can also be used, but use of autogenous material is less likely to produce postoperative aseptic meningitis.6 If clips were used on the midline occipital sinus, they can be removed as the dura is sutured. The suture line may be covered with thrombin-soaked Gelfoam. If a craniotomy was performed, the bone flap can be secured with wires, plates and screws, or sutures. Alternatively, the defect can be covered with a titanium screen held in place by gently compressing the screen and allowing it to insert itself between the dura and inner margins of the bony defect. The fascia is closed with interrupted absorbable sutures to approximate the muscle and fascia (Fig. 31-22). If the fascia is dried and difficult to approximate, the skeletal fixation apparatus can be loosened and the neck extended to facilitate closure. An adequate amount of tissue must be left at the superior fascial flap to prevent buttonholes at superior nuchal line. The scalp is then closed in layers, ending with a subcutaneous reapproximation using interrupted absorbable sutures with inverted knots. If in the sitting position, all layers should start from the caudal end of the wound so that the tails do not hang in the way. The wound is then closed with sutures or staples (Fig. 31-23). The wound is covered with a sterile dressing and the patient extubated in a supine position.
Complications
Hydrocephalus is common with fourth ventricular tumors, and is one of the most significant causes of morbidity and mortality associated with these tumors.7,8 In the past, many patients with tumors and hydrocephalus underwent temporizing preoperative shunting to treat hydrocephalus and prevent pseudomeningocele, CSF leak, and meningitis from fistula. However, more recently it has been observed that shunting is associated with many complications, and the increased incidence of subdural hematoma, infection, and brain-stem compression from upward herniation may outweigh its benefits.9–12 Also, the advent of advanced radiographic imaging has allowed diagnosis of fourth ventricular tumors much earlier than before, when patients were frequently moribund with dehydration and malnutrition from vomiting and hydrocephalus needed to be urgently treated. Today, only about 10% to 20% of patients with cerebellar and posterior fossa tumors require permanent shunting,7,8,13 and most of these have slow-growing tumors such as astrocytoma since more acute tumors distend the ventricles for a short period of time and do not allow outlet adhesions to form. Risk factors for shunt dependence include younger age, larger preoperative ventricle size, and more extensive tumors. In many cases, preoperative high dose steroids will produce satisfactory improvement in hydrocephalus. Otherwise, an appropriate alternative to shunting is perioperative external ventricular drainage,14 especially if a patient presents lethargic or obtunded. This allows for precise pressure monitoring and control of drainage rate to prevent upward herniation and, if continued postoperatively, clearance of debris, proteinaceous blood, and air from the operation. Although external ventricular drainage does reduce the necessity to use permanent shunts, the infection rate may be as high as 10%, so it should be used judiciously. If a shunt is required for a malignant tumor, there may be an increased risk of extraneural metastasis through the shunt tubing (especially to the peritoneum),15 although some studies have suggested that such metastases may occur as often in patients without shunts.16
Pneumocephalus in the ventricles and subdural space is common after fourth ventricular surgery, especially when patients are operated in the sitting position,17 although it also occurs after prone operations. It is much more common when patients have preoperative hydrocephalus, and frequently results from overzealous drainage of CSF through an external ventricular drain intraoperatively. Since nitrous oxide can diffuse into air filled spaces, it is possible that nitrous oxide contributes to tension pneumocephalus, although this is controversial. If tension pneumocephalus is recognized intraoperatively, the patient should be placed in Trendelenburg position and the operative bed irrigated to replace air with the irrigating fluid. Symptomatic postoperative tension pneumocephalus can be treated with a small frontal burr hole to relieve the pressure caused by the trapped air. Intraventricular air may cause ventriculoperitoneal shunt malfunction due to airlock.
Aseptic meningitis, also called posterior fossa fever, is a rare occurrence after posterior fossa surgery, especially for epidermoids or dermoids that rupture intraoperatively leaking cholesterol cyst fluid, although it also occurs after resection of astrocytoma or medulloblastoma. It may be a presenting symptom preoperatively but much more common as a postoperative complication.18,19 Patients usually present about 1 week after surgery with fever, headache, irritability, and CSF pleocytosis. It can be difficult in some cases to differentiate aseptic meningitis from true bacterial meningitis, which should always be carefully excluded before treating for aseptic meningitis. The condition resolves with steroid or anti-inflammatory treatment and serial lumbar punctures to remove CSF.
The “posterior fossa syndrome,” also called posterior fossa mutism or pseudobulbar palsy, is characterized by the delayed onset of mutism, emotional lability, and supranuclear lesions that occurs within a few days after midline posterior fossa operations.20 The syndrome has been seen in as many as 15% of intraventricular approaches to lesions near the brain stem, but has also been described with supracerebellar infratentorial approach to the pineal region and retromastoid lateral cerebellar approach to the side or front of the brain stem. Patients present with global confusion, disorientation, combativeness, paranoia, or visual hallucinations. They are generally alert and will follow simple commands, but will sometimes refuse to speak or present scanning speech. Orofacial apraxia, drooling, dysphagia, pharyngeal dysfunction, and flat affect are common, but there is no actual weakness, hence the term pseudobulbar palsy. Because of the delay in onset, it has been suggested that edema from operative manipulation may play a role, for example through transmission of retractor pressure from the medial cerebellum through fiber pathways along the middle and superior cerebellar peduncles into the upper pons and midbrain. There are no consistent neuropathologic findings, and most patients have some improvement over several weeks to months.20–22
Ipsilateral limb ataxia, dysmetria, dysdiadokinesis, and hypotonia usually results from damage to the cerebellar hemisphere, especially the dentate nucleus, which is located along the superolateral margin of the roof of the fourth ventricle adjacent to the upper pole of the tonsil. Most injuries to the dentate nucleus occur during dissection of a hemispheric tumor. Retraction during dissection of the superior vermis can injure the superior cerebellar peduncle (which) producing similar symptoms. Unless the dentate is completely ablated, most patients recover well within a few months with only minor residual intention tremor that does not interfere with motor development.
Acute urinary retention is an uncommon complication of dissection of the fourth ventricular floor near the striae medullaris, presumably due to injury to the pontine micturition center in the pontine tegmentum, the structure that integrates the cortex with sacral and pelvic sensory pathways that apprise bladder filling status.23 Patients with this condition demonstrate inability to initiate voiding in spite of a full bladder with high intravesicular pressure. Since the pontine micturition center is deep in the pons near the reticular activating system, this symptom is usually associated with a disturbance in sensorium, but can occur in conscious patients. It is usually reversible but does not respond to detrusor augmenting agents or alpha-adrenergic blockers. Patients are best managed by intermittent catheterization.
Patients treated with radiation sometimes have significant learning disabilities,24 and should undergo follow-up neuropsychiatric evaluation. Radiation treatment has also been associated with endocrine dysfunction, growth dysfunction, hypothyroidism, delayed or precocious puberty, and secondary malignancy.25 Patients that have extensive laminectomies are predisposed to development of swan-neck deformity, and should be kept in a soft cervical collar for 6 to 8 weeks until the paraspinal muscles reattach and monitored with cervical spine x-rays every few months for a few years to check for spinal deformities.
Specific Tumors
Medulloblastoma
The term “medulloblastoma” was initially introduced by Bailey and Cushing who noted the highly cellular architecture of small round basophilic cells that showed various degrees of differentiation along neuronal and glial lines. They assumed that the cell of origin (the “medulloblast”) was a primitive cell capable of both neural and glial differentiation.26 More recently, it has been theorized that these are related to tumors with similar histology in other locations, such as the pineal gland (pineoblastoma), ependyma (ependymoblastoma), retina (retinoblastoma), and elsewhere (neuroblastoma). The term “primitive neuroectodermal tumor” (PNET) was used to describe these tumors. A medulloblastoma would therefore be described as a PNET of the fourth ventricle.
Medulloblastoma is the most common malignant primary brain tumor in children, accounting for 20% to 25% of all childhood primary brain tumors and 40% of all childhood posterior fossa tumors.27,28 Peak incidence is 3 to 5 years of age; half of all medulloblastoma patients are under 10 years of age at diagnosis, and three quarters are under age 15. Medulloblastoma is uncommon in infancy, and less than 5% of patients present under 1 year of age. There is a second peak between 20 and 40 years so that medulloblastoma accounts for 5% of all adult posterior fossa tumors and 1% of all adult brain tumors. Adult medulloblastomas are more likely to be hemispheric than midline, likely due to the lateral migration of cells of the granular layer of cerebellum from the inferior medullary velum. Adult medulloblastomas are also more likely to be cystic or necrotic, have poorly defined margins and less contrast enhancement.29 They may even involve the cerebellar surface and resemble meningiomas. There is a slight male preponderance in most clinical series, and for reasons that are unclear, medulloblastomas have a significantly higher incidence in North America than elsewhere in the world.30 Medulloblastomas frequently metastasize in the subarachnoid space, and some dissemination is evident in 20% to 30% of all patients and 50% of young patients at diagnosis.31,32 Familial medulloblastoma has been reported.33
Medulloblastomas grow quickly, so onset of symptoms is usually fairly acute; most patients are symptomatic less than 2 months before the tumor is diagnosed, and very few report symptoms for longer than 6 months. Most patients initially experience symptoms of increased intracranial pressure from CSF obstruction. Symptoms typically begin with intermittent headache (often worst in the morning) followed by vomiting and eventually gait problems.34 Gait difficulties include wide-based gait and inability to tandem walk; these findings are often subtle and not appreciated by the child or the parents but frequently alert the physician to the presence of a neurologic lesion. Clinical signs include ataxia for midline tumors or dysmetria/dysdiadokinesia for lateral tumors. Most patients have papilledema by the time they present for evaluation. Less common signs include diplopia from abducens palsy, facial paresis or lower cranial nerve palsy from tumor invasion, or head tilt from tumor extension into the upper spinal canal or impaction of the cerebellar tonsils at the foramen magnum against the first two cervical nerve roots. Since medulloblastomas can metastasize along the subarachnoid space, some patients present with cranial or spinal nerve root symptoms from distant metastases or even seizures from cortical metastases. Patients who present with signs of metastatic disease have a limited life expectancy.
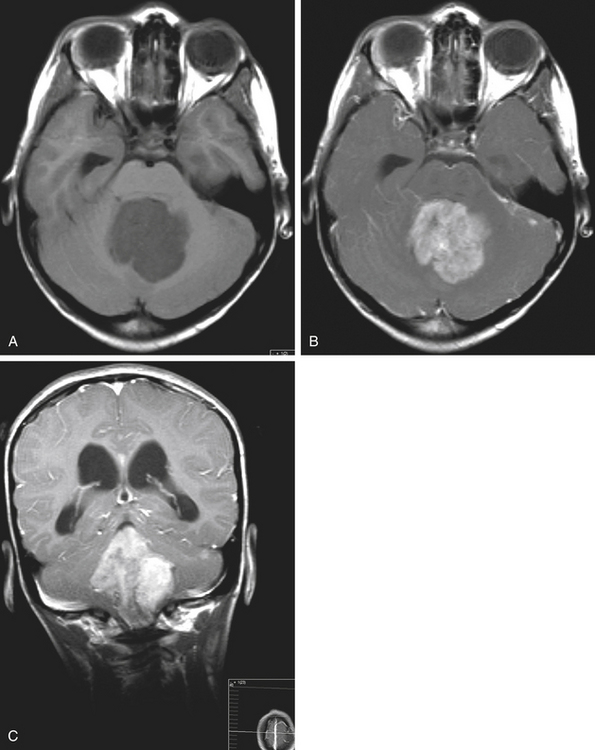
Medulloblastomas usually appear as midline solid tumors on neuroimaging, although cystic change is sometimes observed. Eighty-five percent are midline vermian lesions, usually arising from the vermis or inferior medullary velum and growing into the fourth ventricle, sometimes appearing to be entirely intraventricular.32 Adult lesions are much more likely to be in a lateral location. CT demonstrates a homogeneous hyperdense lesion that enhances intensely and diffusely after contrast administration with occasional heterogeneously enhancing regions due to necrosis, although rarely medulloblastomas do not enhance at all on CT.35 Calcification will be apparent in 10% of medulloblastomas,36 but presence of calcium or cystic change is more typical of ependymomas. On MRI, the lesion is hypointense to isointense to the brain on T1 and hyperintense or hypointense on T2 with heterogeneous signal due to microcysts, necrotic cavities, tumor vessels, or calcification (Fig. 31-24). The tumor displays irregular enhancement with MRI contrast material,36,37 and enhanced MRI will disclose small cortical or basal metastases in 5% to 10% of cases. The sagittal MRI can help delineate the relationship of the tumor to the vermis, midbrain tectum, vein of Galen, and cervicomedullary junction. Additionally, sagittal MRI can differentiate true intraventricular tumors from extraventricular vermian tumors: intraventricular tumors will widen the aqueduct and displace the quadrigeminal plate posterosuperiorly, while dorsal lesions will kink the quadrigeminal plate giving it a C-shaped appearance.38 Infrequently, the tumor will be seen to extend out of foramen of Luschka, but this is far more typical of ependymomas. In young children, medulloblastomas are often radiographically indistinguishable from ependymomas by radiographic appearance alone. Because of the frequency of craniospinal metastases, all patients should get a contrasted MRI of the entire craniospinal axis.
Grossly, medulloblastomas are discrete, soft tumors, although adult lesions tend to be firmer and more adherent to leptomeninges. Medulloblastomas can be divided into two broad histologic patterns: classical and desmoplastic. Classical medulloblastomas, which make up three quarters of the total, are seen to have dense, diffusely monotonous sheets of cells with intensely basophilic nuclei and scant cytoplasm (“small round blue cells”). There is regional variability in the size and shape of cells, number of mitoses, and appearance of nuclei, and necrosis is common. Desmoplastic medulloblastomas have a higher proportion of fibrous stroma associated with the perivascular collagen skeleton of tumor. Sometimes there are uniform compact lines of cells around islands of relative hypocellularity; when this is seen, the compact rims stain heavily with reticulin and the hypocellular areas stain for GFAP. Occasionally individual cells resembling oligodendrocytes are seen, having perinuclear halos and staining with tubulin and synaptophysin. The desmoplastic variant is more common in older patients. In young patients location is not associated with histology, but older patients are more likely to have desmoplastic histology in more lateral tumors.39 In both histologic patterns there are occasionally neuroblastic areas with histology similar to neuroblastomas with Homer-Wright rosettes (rings of nuclei surrounding a central zone of fibrillary processes) and perivascular pseudorosettes that resemble those in ependymomas except that they do not stain for GFAP. Mature ganglion cells are sometimes seen, although it is controversial whether these represent further neuronal differentiation or engulfing of deep cerebellar nuclei by the tumor. Also seen are islands of glial development characterized by clusters of GFAP-staining cells with pink cytoplasm commonly seen as circular whirls of cells with bipolar processes. These may represent entrapped or reactive astrocytes.
Even with a vermian incision, it is seldom possible to expose the entire tumor. Therefore, the next step is to debulk the central portion of the tumor using blunt or sharp dissectors with microscissors and microsuction aspiration. Desmoplastic tumors cannot be aspirated with microsuction, so ultrasonic aspiration can be used for these tumors, but this must be used with caution around the brain stem because of increased destruction of tissue. Bleeding is controlled with bipolar cautery. To expedite removal of the tumor and minimize blood loss, the tumor can be divided into four quadrants; as soon as bleeding becomes troublesome from one quadrant, a micropatty is placed there and attention turned to a different quadrant, and so on. By the time the dissection returns to the first quadrant the bleeding will have slowed enough to allow continued resection. After the tumor has been debulked, the shell of tumor is carefully stripped off the brain stem from inferior to superior using a small brain retractor and separated from the brain stem using cottonoid patties. Cottonoid patties should be placed along the fourth ventricular floor as early as possible to protect the brain stem, and it is important to constantly ensure that the trajectory is correct to prevent diving into the brain stem at an angle. When the brain stem is protected with a cottonoid patty, the inferior half of the tumor can then be safely removed. The tumor is rarely adherent or invasive into the brain stem, but when it is, gross total resection should not be attempted due to the risk of permanent cranial nerve defects. Rather, any focal areas of adhesion are separated using bipolar cautery and covered with a cottonoid. Later, when the tumor is entirely removed, the residual tumor tissue can be carefully aspirated parallel to the plane of the fourth ventricular floor until only a thin lining remains, and this lining can be coagulated with bipolar cautery to reduce the viability of remaining cells.
The next step is to resect the lateral and anterior portions of the tumor. The lateral and superior attachments of the tumor usually blend with the paravermian brain so there is seldom a plane between tumor and normal brain. As a result, most residual tumor fragments are left in this area. Since there are minimal postoperative neurologic deficits that result from removing a thin rim of cerebellum, the dissection should be carried out on the brain side of the brain-tumor interface so that all tumor is resected and hemostasis is easier to obtain. At the end of the dissection there will be a bed of clean white brain. The lateral dissection is extended onto the ependymal surface, where the tumor attaches to the cerebellum, and this margin is defined upward and forward until the dilated caudal aqueduct is reached, producing a conical dissection field. Finally, the anterosuperior tumor pole is removed, leaving the tip of the tumor covering the opening of the caudal aqueduct until the end of the operation so that no blood from the dissection will enter the lateral or third ventricles.