Chapter 108
Lower Extremity Arterial Disease
General Considerations
Hasan H. Dosluoglu
Based on a chapter in the seventh edition by John V. White
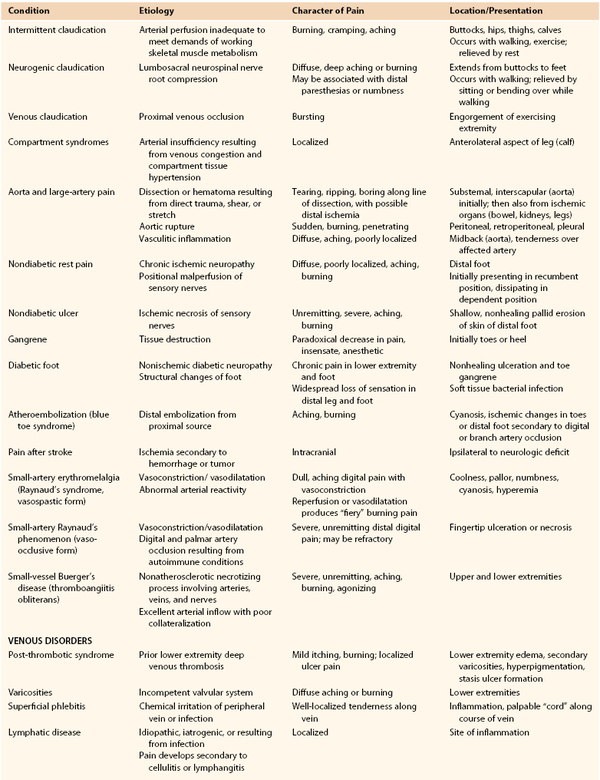
Chronic lower extremity ischemia due to peripheral arterial disease (PAD) is the most common cause of walking disability seen by vascular specialists. The manifestations of chronic lower extremity ischemia often include pain (Table 108-1) produced by varying degrees of ischemia, ranging from no or atypical leg symptoms to typical exertional muscular pain (intermittent claudication, IC) to ischemic rest pain. Patients may have more than one cause for their extremity pain, making diagnosis and management more difficult (see Chapter 14). The challenge for the vascular specialist is to recognize the presence of lower extremity ischemia, quantify the extent of local and systemic disease, determine the degree of functional impairment related to PAD, identify and control modifiable risk factors, and establish a comprehensive treatment program.
Classification
Claudication
The typical patient with IC experiences calf symptoms ranging from fatigue to aching while walking. Pain or discomfort may also occur in the thigh or buttock. The pain sensation results from ischemic neuropathy involving small unmyelinated A delta and C sensory fibers and a local intramuscular acidosis from anaerobic metabolism enhanced by the release of substance P.1 The symptoms of intermittent claudication are alleviated by a brief period of rest, after which the patient can resume walking. Initially, the symptoms do not occur with regularity; they occur intermittently when walking, and the distance walked before symptoms are noticed is generally similar on different outings. As the process progresses, symptoms occur more frequently and after shorter distances.
Asymptomatic patients with a reduced ankle-brachial index (ABI) but no symptoms may have significant impairment of leg function when tested objectively. Among 460 patients with PAD, 91 had no symptoms; of these, 28 were less active and appeared to control their symptoms through a reduction in walking speed and distance, whereas 63 remained active, walking more than 6 blocks a week.2 When subjected to a 6-minute walking test, however, the 63 active patients performed in a manner similar to claudicants, walking slightly farther but with a slower maximal velocity. Thus some patients may be asymptomatic because of their poor medical condition and functional capacity. McDermott et al3 compared 72 asymptomatic patients with PAD to those with claudication (n = 215) and 292 with no PAD. They found that asymptomatic subjects with PAD had worse functional performance, worse quality of life, and more adverse calf muscle characteristics compared with persons with IC, as well as with the sedentary, asymptomatic, age-matched group of non-PAD persons. This underscores the impact of PAD even in asymptomatic patients who limit their activity to control symptoms or because of other medical illness.
Disease Location
Claudication often results from a single level of arterial disease, such as the iliac artery or the superficial femoral artery, but can result from multilevel disease. Collateral vessels can reconstitute the artery distal to a single site of stenosis or occlusion and provide distal flow. Symptoms of claudication associated with PAD usually manifest in the muscle groups below the hemodynamically significant lesion. Three major patterns of arterial obstruction are possible: inflow disease, outflow disease, and a combination of the two. Inflow disease refers to lesions in the suprainguinal vessels, most commonly the infrarenal aorta and iliac arteries. Occlusive lesions of the infrarenal aorta or iliac arteries commonly lead to buttock and thigh claudication. In men, if the stenoses or occlusions are bilateral and are proximal to the origins of the internal iliac arteries, vasculogenic erectile dysfunction may be present as well (see Chapter 82).
Although buttock and thigh claudication may be the first symptoms, with continued ambulation, these patients may exhibit classic symptoms of intermittent calf claudication resulting from inadequate perfusion of the entire leg while walking. Outflow disease consists of occlusive lesions in the lower extremity arterial tree below the inguinal ligament, from the common femoral artery to the pedal vessels. Superficial femoral artery stenosis or occlusion is the most common lesion associated with intermittent claudication, which leads to calf discomfort with ambulation and relief with rest. No specific thigh or foot symptoms are associated with superficial femoral artery occlusion. Because the deep femoral artery provides collateral circulation to and reconstitution of the popliteal artery, isolated superficial femoral artery occlusion without distal disease is rarely the cause of more advanced forms of ischemia. Popliteal and tibial artery occlusions are more commonly associated with limb-threatening ischemia, owing to the paucity of collateral vascular pathways beyond these lesions. As isolated lesions, they are usually not the cause of IC and become clinically significant in patients with tissue loss. They are typically seen in older adults and in patients with diabetes and end-stage renal disease. Long-term corticosteroid therapy has also been reported to be associated with a distally accentuated, calcifying peripheral atherosclerosis, inducing arterial incompressibility comparable to patients with renal failure or diabetes.4 Patients with a combination of inflow and outflow disease may have widespread symptoms of IC affecting the buttock, hip, thigh, and calf. These symptoms frequently begin in the buttock and thigh and then involve the calf muscles with continued ambulation; however, they may appear in reverse order if the distal disease is more severe than the inflow disease. Severe combined inflow-outflow disease may result in limb-threatening ischemia.
In a review of 400 patients with PAD who underwent a first digital subtraction arteriogram of the lower limbs, Aboyans et al5 found that proximal PAD was associated with greater prevalence of male sex and smoking, whereas more distal PAD was associated with older-age, diabetes, hypertension, and renal failure (P <.05). They found that proximal PAD was associated with a worse prognosis, after adjustments for age, sex, cardiovascular disease, critical leg ischemia, and treatments, but these results need to be confirmed in a more general population of patients with PAD.
Nonatherosclerotic Causes of Claudication
Intermittent claudication in younger individuals may be caused by popliteal artery entrapment syndrome or adventitial cystic disease of the popliteal artery (see Chapter 115), chronic compartment syndrome (see Chapter 163), or kinking or endofibrosis of the iliac arteries. The pain of popliteal entrapment, produced by extrinsic compression of the popliteal artery by the gastrocnemius muscle during leg movement, is similar to that of IC and has the same pathophysiologic mechanism as that associated with PAD.6 Popliteal adventitial cystic disease produces similar symptoms. Chronic compartment syndrome causes exercise-related discomfort only in the anterolateral aspect of the calf. The cellular basis for the anterior compartment muscular pain associated with chronic compartment syndrome is ischemia resulting from diminution of the muscular arteriovenous pressure differential owing to venous congestion and compartment tissue hypertension.7 Iliac artery endofibrosis with kinking is characterized by thickening of vessel intima due to subendothelial accumulation of loose connective tissue containing variable amounts of collagen, elastin, and smooth muscle cells, resulting in progressive stenosis and impaired flow, and has been most commonly described in competitive cyclists. It causes mostly unilateral pain, cramping, or numbness, which may become apparent only at maximal exercise.8 Nonatherosclerotic causes of IC are listed in Box 108-1.
Critical Limb Ischemia
Critical limb ischemia (CLI) is the most severe form of PAD and represents approximately 1% of the total number of patients with PAD.9 The natural history of CLI differs significantly from that of claudication. CLI is associated with a higher risk of limb loss in the absence of revascularization, whereas claudication rarely progresses to the point of requiring amputation. In patients with CLI, the arterioles become maximally vasodilatated and insensitive to vasodilatory stimuli as a result of the chronic exposure to vasorelaxing factors. These dilatated peripheral arterioles have decreased wall thickness and cross-sectional area, leading to edema, which is aggravated by keeping the limb dependent. Chronic ischemia also results in changes in structure and function of endothelial cells, and coupled with platelet activation, leukocyte adhesion result in microthrombi formation in the capillaries. All these changes result in impaired tissue oxygen exchange at the capillary level.10,11
The common major manifestations of CLI are rest pain and ischemic ulceration or gangrene of the forefoot or toes, representing a reduction in distal tissue perfusion below resting metabolic requirements. Rest pain is usually described as a burning sensation or as an uncomfortable coldness or paresthesia of sufficient intensity to interfere with sleep. The ischemic neuropathy in CLI may also cause numbness, and since many patients also have diabetes, it may be difficult to determine how much of the neuropathic changes are caused by ischemia alone.12 The discomfort is worsened by leg elevation, because of the loss of the gravitational pull of blood to the foot; it is relieved by placing the limb in a dependent position, such as dangling it off the side of the bed. In patients with typical ischemic rest pain localized to the forefoot, occurring with elevation and relieved by dependency, the clinical diagnosis is objectively confirmed by hemodynamic measurements such as systolic ankle pressure less than 50 mm Hg, toe pressure less than 30 mm Hg, or ABI less than 0.40. It is important to note that patients with diabetic foot ulcers may have inadequate blood flow for healing even with perfusion levels that exceed these criteria for CLI. In fact, the term CLI was never intended to be applied to patients with diabetes and foot wounds.13
Ischemic ulcers usually represent the effect of repetitive soft tissue trauma, often very mild in degree, with erosion of the overlying skin. Skin repair is hampered by inadequate tissue perfusion, oxygenation, and cellular replication. Arterial ulceration in a nondiabetic patient is characterized by a shallow, nonhealing, pallid erosion of the skin in the distal foot—in a distribution similar to that of rest pain. The pain of such ulcerations, described as aching or burning, is often unremitting and severe and is occasionally refractory to even high-dose oral narcotic analgesics. It is the result of not only chronic, severe ischemic neuropathy but also actual exposure of the sensory nerves in the skin at the site of the ulcer. Diabetic foot ulcerations are broadly divided into ischemic, neuroischemic, and neuropathic ulcers. In recent studies, more than 50% of diabetic foot ulcers are of ischemic or neuroischemic origin.14,15 Therefore ischemia needs to be excluded in all ulcers using objective assessment, since PAD is the most important limiting factor for healing of ischemic or neuroischemic diabetic foot ulcers. In some patients, arterial perfusion may only be decreased to a specific region of the foot (angiosome, see Chapter 116), which may require increasing the flow to that specific angiosome to expedite ulcer healing.16
Ischemic gangrene occurs when resting limb blood flow is insufficient to maintain cellular viability. Tissue death inexorably extends to the junction of threshold blood flow for tissue viability. Initially, the pain may be severe, resulting from not only ischemic neuropathy but also ischemic injury of the skin and subcutaneous sensory nerves, osteomyelitis, and ascending infection. As the course of ischemic necrosis progresses, pain may actually decrease as a result of complete ischemic death of the nerves and other pain-producing tissues. Progression to gangrene occurs in 40% of patients with DM, compared with only 9% in nondiabetic patients with CLI.17
Limb-threatening ischemia usually requires the presence of severe PAD at two or more levels, the additive effects of which severely limit flow through collateral beds and result in profound distal ischemia. The pattern of arterial obstruction often affects sequential vascular beds, such as femoropopliteal and infrapopliteal arteries, but it may affect parallel beds, such as superficial femoral and deep femoral vessels. Both patterns prevent collateralization and reconstitution of the more distal arterial tree. In patients with diabetes, arterial occlusive disease primarily affects the crural and pedal arteries.18
Epidemiology
The prevalence of PAD has been the subject of numerous investigations over the past several decades19–22 The best method of assessing the prevalence of chronic lower extremity arterial occlusive disease is to record the ABI and correlate it with risk factors.
Prognostic Value of Ankle Brachial Index
ABI results are recommended to be reported with noncompressible values defined as greater than 1.40, normal values 1.00 to 1.40, borderline 0.90 to 0.99, and abnormal 0.90 or less.23 The ABI correlates well with the mortality risk associated with PAD, regardless of whether leg symptoms are present. Feringa et al performed a longitudinal study of 3209 subjects followed for 8 years after recording baseline resting and postexercise ABIs. In this study, lower resting ABI values, lower postexercise ABI values, and a greater drop in resting ABI were associated with a higher incidence of death.24 In a cohort of 6880 unselected subjects ≥65 years old who were monitored for over 5 years in the German Epidemiological Trial on Ankle Brachial Index Study Group,25 836 had asymptomatic PAD (ABI <0.9) and 593 had symptomatic PAD. The composite endpoint of all cause death, myocardial infarction or stroke was similar in symptomatic and asymptomatic patients with PAD, both of which carried significantly higher risk than subjects without PAD. Because 21% of subjects had symptomatic or asymptomatic PAD, the ACCF/AHA 2011 writing group recommended ABI diagnostic screening for patients ≥65 years or for patients ≥50 with a history of smoking or diabetes.23
Prevalence of PAD
Prevalence Based on ABI
In the United States, a comprehensive effort to establish the prevalence of PAD using ABI was undertaken in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2000,26 involving 9000 individuals 40 years of age or older. ABIs and a complete data set were available for analysis in 2174 participants. The overall prevalence of PAD (defined as an ABI <0.90) was 4.3% (95% confidence interval [CI], 3.1% to 5.5%). Although prevalence was slightly higher in men than in women, the prevalence dramatically increased with age, rising from 0.9% in those younger than 50 years to 14.5% in those 70 years or older (Fig. 108-1). Statistically significant associations between PAD and the common risk factors of hypertension, diabetes, hypercholesterolemia, and smoking were also noted.
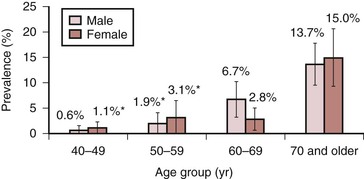
Figure 108-1 Prevalence of peripheral arterial disease by age and gender in adults 40 years and older, United States, 1999-2000 (n = 2174). (Redrawn from Selvin E, et al: Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation 110:738-743, 2004.)
Prevalence Based on Demographics
The relationships of PAD to age, gender, race, and ethnicity have been confirmed by several studies. In the AHA writing group’s meta-analysis,27 the prevalence of PAD was noted to increase with age for both men and women. Using the U.S. census data from 2010, they found that the number of females with PAD was higher among U.S. adults ≥40 years of age. Data adapted from the ABI Collaboration Study, including 480,325 person-years of follow-up of 24,955 men and 23,339 women from the general population who had ABIs measured at baseline and during follow-up, showed that although the ABI correlated with total and cardiovascular mortality rates, which were similar in women compared with men, the risks of morbidity and mortality were increased in women with ABI values either <0.90 or ≥1.40.28
In a primary care setting, 403 patients stratified by race and gender were evaluated with ABIs to determine the prevalence of PAD.29 Study subjects included white, black, and Hispanic women and men. No gender differences were noted, but as with the NHANES data, black women had a significantly greater prevalence of PAD than did white or Hispanic women. A follow-up study using NHANES data from 1999 to 2004 reevaluated the prevalence of PAD in the general population and in ethnic subpopulations.30 Overall, non-Hispanic black men and women (19.2% prevalence) and Mexican American women (19.3% prevalence) had a higher prevalence of PAD than did non-Hispanic white men and women (15.6% prevalence). These studies clearly demonstrate that there is a high prevalence of lower extremity PAD in the United States, affecting an estimated 8 to 12 million people. Further, PAD is now reported to be associated with equal morbidity and mortality and comparable, or possibly higher, cost compared with coronary heart disease and stroke.27,31 The prevalence is higher in some ethnic subpopulations and in those with uncontrolled risk factors, including hypertension, smoking, hypercholesterolemia, diabetes, and renal failure, although in the German Epidemiological Trial,25 48% of patients with asymptomatic PAD were reported to have never smoked, 66% did not have diabetes, and 15% to 16% did not have hypertension or hyperlipidemia. Because of PAD’s high prevalence and substantial mortality risk, even in the absence of symptoms, it is essential to identify and treat patients with PAD. Adding reduced ABI to traditional risk factors increases the sensitivity of the identification of patients with moderate to high risk of cardiovascular mortality.
Prevalence Based on Risk Factors
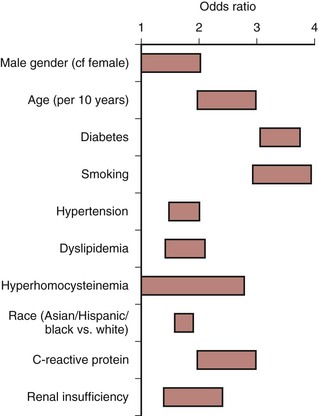
Hypertension increases the risk of developing symptoms of IC 2.5-fold in men and 3.9-fold in women,32,33 and is present in 55% of patients with PAD.34 The relationship between diabetes and IC has also been well documented.32,33,35 PAD prevalence is 20% to 30% higher in diabetics than in the general population,36 and the risk of developing PAD correlates with the severity and duration of diabetes.37 Patients with diabetes are more likely to have symptomatic PAD, with a 3.5-fold increased risk in men and an 8.6-fold increased risk in women.38 Metabolic syndrome is estimated to be present in at least 25% of the population.39 This syndrome is defined as having three or more of the following: blood pressure elevation (≥130 mm Hg/≥85 mm Hg), triglyceride count ≥150 mg/dL, high-density lipoprotein count ≤50 mg/dL for women or ≤40 mg/dL for men, fasting blood glucose ≥110 mg/dL, and abdominal obesity (BMI ≥30 kg/m2 or waist circumference ≥102 cm in men, ≥88 cm in women). An analysis of data from three National Health and Nutrition Examination Surveys (NHANES, 1999-2004) involving 5376 asymptomatic participants 40 years and older showed that 38% of the population with PAD also had metabolic syndrome, and the prevalence of PAD (ABI <0.9) was 7.7% and 3.3%, respectively in those with and without metabolic syndrome.40
Cigarette smoking is a long-established stimulus for atherosclerosis and increases the risk that PAD will develop in men and women.32,35 A lifetime smoking history exceeding 25 pack years has been reported to be associated with increased risk of PAD (HR 2.72) compared with those who never smoked41; the risk is higher in women than in men, and smoking cessation is associated with substantial risk reduction for development of PAD.42 The severity of arterial occlusive disease is proportional to the number of cigarettes smoked,43 and each additional risk factor independently increases the risk of developing symptomatic PAD (Fig. 108-2).
Natural History
Asymptomatic Disease
Patients with asymptomatic PAD may eventually develop symptoms of claudication or may demonstrate little progression of their disease. The Edinburgh Artery Study found that patients with asymptomatic PAD had no statistically significant drop in ABI over the 5 years of observation.44 Regardless of whether symptoms are present, individuals with PAD, identified by an ABI less than 0.90, have higher morbidity and mortality than age-matched controls with normal ABIs. The risks are inversely related to the amount of physical activity the patient undertakes each day. Evaluating the natural history of 460 patients with ABI-proven PAD, investigators noted that reduced physical activity correlated with increased mortality and cardiovascular events.45 Therefore patients who attempt to control or eliminate their lower extremity PAD symptoms by reducing their walking actually worsen their risk of myocardial infarction (MI), stroke, and death. This asymptomatic group of patients with PAD should be managed medically in the same way as those with symptoms of IC.
Impact of Female Gender
Women with PAD were reported to experience faster functional decline than men with PAD. McDermott et al46 assessed 380 men and women with PAD using a 6-minute walk test and assessment of mobility disability at baseline and yearly for up to 4 years, and used CT to assess calf muscle characteristics biannually. They found that at 47 months of follow-up, women with PAD were more likely to become unable to walk for 6 minutes continuously, had a higher incidence of mobility loss, and had faster declines in walking capacity compared with men. The more rapid deterioration in women with PAD was attributed to the poorer functional performance and smaller baseline calf muscle mass, resulting in women being closer at baseline to the thresholds for immobility.
Impact on Future Health
The presence of PAD in asymptomatic patients was also found to be a significant risk factor for future disability. In the Cardiovascular Health Study of 4705 participants 65 years of age and older who had ABI measured between 1992 and 1993, lower baseline ABI values were found to be associated with increased risk of late-incident mobility disability and activities of daily living disability during a 6-year follow-up.47 Most recently, Leeper et al assessed 725 PAD patients using a customized symptom-limited ramp treadmill protocol between 1997 and 2011 and found that exercise capacity was the strongest independent predictor of death, with each additional MET achieved being associated with age-adjusted 18% and 20% reductions in all-cause and cardiovascular mortality, respectively (P <.001 for both), surpassing all classical risk factors and all measured exercise tests.48
Intermittent Claudication
Impact on Extremity
The natural history of IC is marked by slow progression to shorter walking distances, but it rarely reaches the level of CLI. Only about one fourth of patients with IC ever deteriorate significantly, and deterioration is most frequent during the first year after diagnosis (6% to 9%) compared with 2% to 3% per annum thereafter.49 This is especially true if risk factors are controlled. Of 224 nondiabetic patients with IC followed for 6 years, only 8% of those who stopped smoking progressed to rest pain, whereas 79% of those who continued to smoke developed signs of CLI.50 Similarly, in a long-term study of 1244 claudicants, only insulin-requiring diabetes, low initial ABI, and high pack-years of smoking predicted progression to ischemic rest pain and ischemic ulceration.51 The risk of major amputation is less than 5% over a 5-year period.44
Quality of Life
Reduced independent mobility and the discomfort imposed by IC profoundly impact a patient’s quality of life. The Short Form (36) Health Survey (SF-36), a generic quality-of-life instrument that includes eight domains to assess physical and emotional function, has been used extensively to document the effect of claudication on quality of life.52 In a study of 68 claudicants, scores in all eight domains were reduced compared with nonclaudicants, especially physical function and role limitations due to emotional impact.53 These findings were extended in a community-based study of 53 patients with documented IC and 327 controls without claudication.54 Using the Rose Intermittent Claudication Questionnaire and the SF-36, the investigators noted reductions in physical function, role limitations due to physical dysfunction, role limitations due to emotional dysfunction, and changes in bodily pain, energy, and general health perception in patients with IC. Only social function and mental health appeared to be unaffected. The adverse impact of IC appears to be directly related to walking ability. Limitations on ambulation give rise to broad physical and emotional effects, as documented in a study of 80 claudicants evaluated with the Walking Impairment Questionnaire, SF-36, ABI, and 6-minute walking test.55 The results of the 6-minute walking test correlated well with quality-of-life scores. Patients with shorter walking distances during the walking test had worse scores in the physical function and role limitations due to physical dysfunction.
Association with Systemic Atherosclerosis
The presence of PAD as documented by an ABI less than 0.90 is also a strong marker for the presence of coronary artery disease (CAD) and cerebrovascular disease (CVD). In the PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) study, which assessed 6979 patients aged 70 years or older or aged 50 to 69 years with diabetes or a history of smoking, symptomatic CAD or CVD was identified in 16% of study subjects with an ABI less than 0.90.56 In the Reduction of Atherothrombosis for Continued Health (REACH) Registry,57 which included an international, prospective cohort of 68,236 patients with either established atherosclerotic arterial disease (CAD, PAD, CVD; n = 55,814) or at least three risk factors for atherothrombosis (n = 12,422), the overall cardiovascular death, MI, or stroke rates in 1 year were 4.5% for patients with CAD, 6.5% for patients with CVD, and 5.4% for patients with PAD. The 3-year MI/stroke/vascular death rates in the 32,247 patients in this registry were significantly higher for patients with symptomatic disease when compared with those with risk factors only (12% vs. 6%, P <0.001).58 In another study, the fate of 2777 male claudicants was documented over a 15-year period, and mortality rates of 42% and 65% at 5 and 10 years, respectively, were noted.59 MI accounted for 66% of the deaths among the 1363 claudicants who died during the study period. The risk of cardiac or cerebrovascular disease increases with lower ABI values, as confirmed by the Atherosclerotic Risk in Communities Study.60
Thus the natural histories of asymptomatic PAD and IC are similar and marked by a significantly elevated risk of fatal cardiac and cerebrovascular events, despite the rather small risk of progression to CLI.
Critical Limb Ischemia
Impact on Extremity
The natural history of CLI is grim; approximately 40% of affected individuals lose their legs and 20% die within 6 months of onset. However, an increasing number of patients with CLI receive some form of active treatment, with over half receiving revascularization, and the amputation rate may be decreasing. An estimation of the primary treatment of CLI patients and their status a year later is shown in Fig. 108-3. Meta-analyses of patients who had popliteal-distal bypass or infrapopliteal angioplasty showed similar limb salvage rates of about 87% at 12 months and 82% at 3 years.61,62 A steady but slow decrease in amputation rates in the last decade has been reported, based on various U.S. national and state databases.63–65 Patients with CLI appear to have a more aggressive form of PAD, with involvement of several segments of the lower extremity arterial tree, especially infrapopliteal vessels. Not all patients with CLI progress through stages of worsening claudication before advancing to the severely ischemic level. In a prospective study on stump healing in 713 below-knee amputations, more than half the patients were noted to have no symptoms 6 months before presenting with CLI that required amputation.66 Because of this unpredictability of development of CLI, interruption of the disease process before the development of CLI is not always possible.
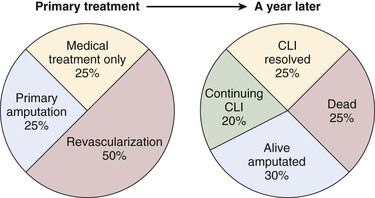
Figure 108-3 The estimate of the initial treatment and status a year later of patients presenting with chronic critical limb ischemia. (Redrawn from Norgren L, et al: TASC II Working Group, Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45:S11, 2007.)
In several studies of patients with CLI due to unreconstructable arterial occlusive disease, the reported natural history is widely variable, with major amputation rates ranging from 14.3% to 46.4%.67–70 These variable outcomes likely reflect inconsistencies in the definition and application of the term CLI in the initial patient cohorts. The risk of major amputation appears to be inversely proportional to the ABI. In a prospective study of 142 patients harboring 169 severely ischemic limbs with ulceration who could not undergo revascularization, only 15% of patients with an ABI greater than 0.50 required major amputation, whereas 34% of those with an ABI less than 0.50 sustained major limb loss at the end of 1 year.71 This abysmal natural history of CLI propels most vascular specialists to attempt to recommend revascularization to reduce the risk of limb loss.
Quality of Life
The traditional methods of assessing outcomes and quality of care in patients with CLI such as survival and limb salvage is increasingly noted to be inadequate, and functional outcomes, such as maintenance of ambulatory status and independent living status, achievement of healed wound status, avoidance of repeat hospitalizations, and interventions, are proposed as more meaningful parameters in these patients. A disease-specific questionnaire for CLI has not been developed; however, the Vascular Quality of Life (VascuQol) Questionnaire, which was designed as a disease-specific tool for patients with PAD, is accepted to be applicable to patients with CLI.72 Using such questionnaires will enable a more comprehensive assessment and patient-oriented approach to patients presenting with CLI, rather than focusing only on amputation-free survival.
Association with Systemic Atherosclerosis
As would be expected given the systemic nature of atherosclerosis, severe PAD is often associated with advanced coronary artery and cerebrovascular disease. CAD has been estimated to be present in approximately half of patients with CLI.73,74 This strong association results in an exceedingly high mortality from MI and stroke among patients presenting with CLI, significantly higher than those with PAD alone. A review of major series reporting the fate of patients with CLI noted that 26% died within 1 year of diagnosis75 and had 5- and 10-year mortality of 50% and 70%, respectively.74,76,77 In patients with diabetes who are known to have a twofold increased cardiovascular mortality compared with nondiabetics, the development of diabetic foot ulcers is associated with even more significant increase in all-cause and cardiovascular mortality.78 In a meta-analysis of eight studies including 17,830 patients with 81,116 person-years of follow-up, diabetic foot ulcer was found to be associated with an increased risk of all-cause mortality, fatal myocardial infarction, and fatal stroke.79