CHAPTER 48 Ebstein Anomaly
The incidence of congenital heart disease ranges between 2.2 and 8.8/1000 live births.1 Cyanotic lesions account for a low percentage of these defects, but result in a significant proportion of congenital heart morbidity and mortality, particularly if left untreated. Characterization of the pulmonary blood flow (PBF) and cardiac chamber morphology remains a fundamental step in evaluating suspected cyanotic congenital heart disease. This chapter will review cyanotic congenital heart disease with cardiomegaly and decreased PBF, specifically focusing on Ebstein anomaly.
EVALUATING THE CYANOTIC PATIENT
MRI, MRA, and CTA are alternative noninvasive modalities to catheterization and may provide comprehensive evaluation of cardiac and pulmonary morphology and function, which is key to diagnosing the cyanotic patient with decreased pulmonary vascularity (Figs. 48-1 and 48-2). Both can generate cardiac chamber volume and qualitative and quantitative functional data. Image postprocessing can readily be facilitated for data sets from both modalities.

 FIGURE 48-1
FIGURE 48-1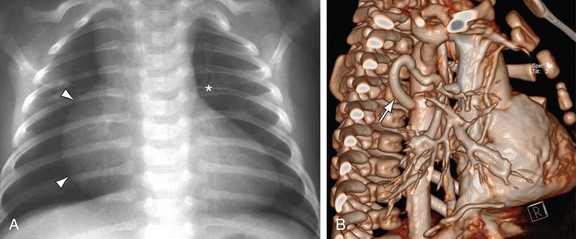
 FIGURE 48-2
FIGURE 48-2