Chapter 72 Disorders of the Cerebellum, Including the Degenerative Ataxias
Cerebellar ataxia can result from a variety of insults to the cerebellum and its connecting pathways. The cerebellum can be the seat of pathology in many well-recognized diseases of the central nervous system (CNS) and may be involved in such processes in isolation or in combination with other structures. Ataxia can either be the major feature of the disease or one of its various clinical signs. Progressive degeneration of the cerebellum and its connections resulting in ataxia can be due to a number of genetic abnormalities, classified as inherited ataxia. Lastly, the term sporadic ataxia or idiopathic ataxia is used for those diseases in which ataxia related to cerebellar degeneration occurs in the absence of a definite genetic or acquired etiology (Klockgether, 2010). In this chapter, we discuss some of the well-defined acquired causes of ataxia, summarize the current understanding of inherited ataxias, and address the issue of sporadic ataxia.
Acquired Ataxias
In many patients, progressive ataxia results from environmental insults and other well-recognized disorders involving the nervous system. In any patient presenting with cerebellar ataxia, such disorders as ischemic or hemorrhagic stroke involving the cerebellum, previous episodes of cerebral hypoxia, primary or metastatic tumors, and CNS demyelinating diseases such as multiple sclerosis can be diagnosed by appropriate history, imaging studies, and other investigations. Many of these diseases tend to have an acute or subacute evolution rather than the chronic course associated with degenerative ataxias. There are, however, diseases that can present with ataxia in which the major imaging abnormality may be an atrophic cerebellum akin to the finding in degenerative ataxias (Table 72.1). Some of these will be briefly discussed next.
| Entity | Diagnostic Process |
|---|---|
Hypothyroidism
Occasionally, patients with hypothyroidism develop a mild dysequilibrium and gait ataxia in conjunction with their systemic symptoms. This is correspondent with pathological changes in midline cerebellar structure (Barnard et al., 1971). Thyroid functions should be tested in patients with progressive ataxia, since replacement treatment may dramatically improve the symptoms (Jellinek and Kelly, 1960).
Toxic Causes
Alcohol
Alcohol remains the major exogenous agent that causes ataxia. A significant proportion of alcoholics have midline cerebellar degeneration at autopsy. Clinically this disease is characterized by a progressive trunk ataxia with gait disturbance of a cerebellar type; upper-limb ataxia, speech difficulties, or eye movement abnormalities occur only in a small proportion of patients. This may reflect the relative sparing of the cerebellar hemispheres. Imaging studies typically reveal vermian atrophy. Chronic alcoholism can also produce significant cerebellar atrophy in the absence of major clinical deficits (Hillbom et al., 1986). Gait ataxia can dramatically improve with abstinence for over a year (Diener et al., 1984).
Chemotherapeutic Agents
Some cancer chemotherapeutic agents are recognized to produce ataxia as a side effect. 5-Fluorouracil (5FU), a fluorinated pyrimidine that acts by incorporating into ribonucleic acid (RNA) and interfering with RNA function, has been used to treat breast and gastrointestinal cancer. Conventional doses of 5FU may cause cerebellar ataxia if there is an abnormality of pyrimidine metabolism in the form of dihydropyrimidine dehydrogenase deficiency. Higher doses of 5FU can also cause a pancerebebellar syndrome that has an acute to subacute evolution (Gottlieb and Luce, 1971).
When cytosine arabinoside (ara-C) is given in high doses (>3 g/m2 for 8-12 doses, as opposed to the conventional 100-200 mg/m2 for 5-7 days), a significant number of patients develop a cerebellar syndrome. Pathologically, this is characterized by loss of Purkinje cells, gliosis, loss of dentate neurons, and spongiform changes (Salinsky et al., 1983). Signs of cerebellar dysfunction mandate immediate cessation of high-dose cytosine arabinoside.
Heavy Metals
Organic mercury poisoning has occurred in epidemic form as a result of contamination from mercury-containing fungicides. Mercury appears to be particularly toxic to cerebellar granule cells and visual cortex and causes a syndrome that includes paresthesia, ataxia, and restricted visual fields. Lead toxicity causes encephalopathy that is especially common in children. It is usually associated with abdominal colic. However, cerebellar signs can be the prominent presentation. Successful chelation therapy may achieve complete neurological recovery (Mani and Chaudhary, 1998). Gait ataxia associated with other signs such as confusion and myoclonus has also been described with bismuth toxicity due to excessive intake of bismuth subsalicylate (Pepto-Bismol) (Gordon et al., 1995). This is reversible over several weeks or months when bismuth intake is stopped.
Anticonvulsants
Transient cerebellar signs associated with supratherapeutic levels of drugs have been seen with many anticonvulsants, especially phenytoin. More persistent ataxia and documented Purkinje cell loss has been primarily seen in epileptics treated with phenytoin for prolonged periods of time. Cerebellar atrophy occurs in phenytoin-treated patients but may not always be associated with overt ataxia. The pathogenesis of this syndrome remains unclear. The various hypotheses include a direct toxic effect of phenytoin, the result of repeated hypoxia related to seizures, the effect of the seizure-related electrical discharge on cerebellar Purkinje cells, and the possibility that both the seizures and the cerebellar pathology are secondary to a unifying underlying pathology such as prenatal injuries. Another intriguing possibility is that both the seizures and the progressive cerebellar syndrome could result from a single underlying gene mutation such as can be seen in SCA10 (Matsuura et al., 2000). It is probably best to avoid phenytoin in an epileptic patient if either ataxia or cerebellar atrophy is present. In a meta-analysis of second-generation anticonvulsants (gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, zonisamide) used as adjunct therapy, all of them were found to increase the risk of imbalance, with the exception of gabapentin and levetiracetam. A dose-response relation was seen with almost all the drugs (Sirven et al., 2007).
Infectious and Transmissible Diseases
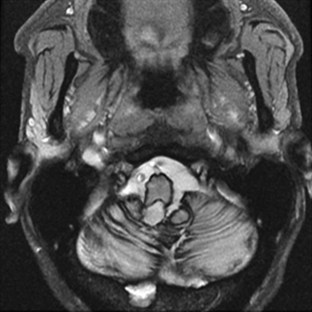
Ataxia can be one of the features of postinfectious encephalomyelitis but usually accompanies a more diffuse cerebral process. A more restricted cerebellar syndrome has been seen in children after a viral syndrome. Connolly et al. (1994) have proposed that this diagnosis be considered when children develop an acute ataxic disorder not associated with a more diffuse process reflected by seizures, meningismus, or obtundation. In most children, this was preceded by a nonspecific viral syndrome or varicella, with a peak incidence at the age of 5 to 6 years. A similar syndrome following Epstein-Barr virus (EBV) or vaccinations occurred in the teenage years. Cerebrospinal fluid (CSF) may show some elevation of protein and a modest mononuclear pleocytosis, and PCR may allow identification of a specific etiology such as EBV. Magnetic resonance imaging (MRI) often reveals signal density changes in the cerebellum (Fig. 72.1). Prognosis for recovery is usually excellent, with residual dysfunction in a distinct minority. However, swelling, hydrocephalus, and herniation have been described in some and may require decompressive surgery (Van Lierde et al., 2004).
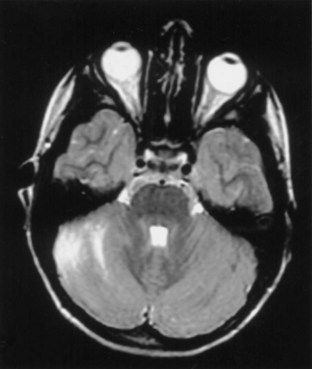
Fig. 72.1 Signal density change in the cerebellum in a child with acute cerebellar ataxia of childhood.
(Courtesy Dr. V. Vedanarayanan, Department of Pediatrics, University of Mississippi Medical Center.)
Similarly, a combination of ataxia, ophthalmoplegia, and other lower cranial nerve palsies can occur as a result of brainstem encephalitis (Bickerstaff encephalitis). There appears to be significant overlap between this disease and the Miller-Fisher variant of Guillain-Barré syndrome. In a report of 62 cases diagnosed as Bickerstaff encephalitis, most had some changes in consciousness level, 40% had Babinski signs, and 60% had tetraparesis; in addition, anti-GQ1 antibody was present in 66%. MRI brain abnormalities occurred only in 30% (Odaka et al., 2003).
Human immunodeficiency virus (HIV) infection can result in many neurological syndromes including ataxia. The majority of patients with ataxia in the presence of HIV infection have discrete, well-recognized lesions such as lymphomas, chronic meningeal infection, progressive multifocal leukoencephalopathy, or toxoplasmosis. Approximately 30% of patients with HIV dementia have an ataxic syndrome at the onset of their illness before cognitive decline begins. Other authors have described isolated progressive cerebellar ataxia that does not evolve into HIV dementia in a small number of patients (Elsheikh et al., 2010; Kwakwa and Ghobrial, 2001; Tagliati et al., 1998). These patients had a rapid evolution of their ataxia leading to chair-bound status in less than a year; MRI revealed cerebellar atrophy. Pathological examination has revealed marked granular cell loss.
Creutzfeldt-Jakob disease (CJD) should be considered in patients presenting with progressive ataxia. CJD is typically a rapidly progressive dementing illness related to the accumulation of mutant prion protein (PrP), which is a posttranslational modification of the normal prion protein. Among sporadic CJD patients, over 70% have ataxia (Parchi et al., 1999). Jellinger et al. (1974) noted that the ataxic variant of CJD began with minor behavior symptoms followed by ataxia. Upper motor neuron signs were frequent; myoclonus occurred only in 25%, and dementia evolved late. Survival among these patients was slightly longer than in typical CJD, with a mean time to death of 16 months and a range of 7 weeks to 8 years. Pathologically, the cerebellum shows striking granule cell loss. A recent review noted that both the iatrogenic and variant form of CJD (human bovine spongiform encephalopathy) usually have ataxia at the onset, as does the inherited form known as Gerstmann-Sträussler-Scheinker syndrome (GSS) (Wadsworth and Collinge, 2007). Prion protein gene mutation analysis is helpful in the familial cases. Iatrogenic cases are usually homozygous for codon 129, and the same codon is all methionine in the variant cases. Tonsillar biopsy for immunocytochemistry for PrP and Western blot studies for abnormal PrPSc (scrapie prion protein) can be useful in the variant cases as well. CSF protein biomarker studies involving 14-3-3, S100b, and tau are often useful in diagnosing CJD (Pennington et al., 2009).
Whipple disease (WD) is a multisystemic infectious disease caused by Tropheryma whippelii. The classic symptoms include diarrhea, weight loss, and abdominal pain. The pathognomic symptoms of CNS involvement are oculomasticatory myorhythmia (OMM) and oculo-facial-skeletal myorhythmia. Many cases of CNS WD are not diagnosed until postmortem (Gerard et al., 2002), but it is postulated that all patients have CNS involvement. Cerebellar ataxia is a common feature of CNS WD. It has been reported to be present in 45% of cases, more common than OMM (Matthews et al., 2005). Since untreated WD is fatal and therapy is available, it is paramount important to consider WD in the differential diagnosis when a patient presents with ataxia along with gastrointestinal symptoms. Those with possible CNS WD should undergo multiple duodenal biopsies if needed. Polymerase chain reaction (PCR) assay of CSF, brain MRI, and a stereotactic cerebral biopsy can also be helpful. Prolonged antibiotic therapy with drugs able to cross the blood-brain barrier is recommended. A regimen with intravenous ceftriaxone sodium, 2 g twice daily for 2 weeks, followed by oral trimethoprim-sulfamethoxazole, double strength, 1 tablet twice daily, achieved good results (Matthews et al., 2005). CSF should be analyzed repeatedly to monitor efficacy. Treatment can be discontinued only when the results of CSF PCR are negative.
Autoimmune Causes
Paraneoplastic Cerebellar Degeneration
Paraneoplastic cerebellar degeneration is a rapidly progressive pancerebellar syndrome that reaches its nadir within a few months of onset. It produces a severe ataxic disease associated with dysarthria and oscillopsia. Diplopia and vertigo may also occur. Many patients also develop other neurological signs including dementia, extrapyramidal signs, hearing loss, and dysphagia. MRI typically shows atrophy of the entire cerebellum, though some high-signal density changes may occur in the deep white matter. CSF usually shows a mononuclear pleocytosis, and oligoclonal bands may be present. It is believed that in many cases the syndrome results from an autoimmune process triggered by the cancer, but evidence for this cannot be found in every patient. Several autoantibodies have been described in these patients. The anti-Yo antibody is usually seen in ovarian cancer and primarily causes a cerebellar syndrome. The anti-Hu (type 1 antineuronal nuclear antibody [ANNA1]) antibody is seen with small-cell cancer of the lung and typically causes a multifocal disorder, the most common of which is a sensory ganglionopathy. Purkinje cell degeneration has been noted in about 25% of patients with anti-Hu antibody. The anti-Ri (ANNA2) antibody that recognizes two closely related neuronal RNA-binding proteins designated Nova-1 and Nova-2 has been seen in patients with a opsoclonus-myoclonus-ataxia syndrome in the setting of breast cancer and non–small cell lung carcinoma (Luque et al., 1991; Musunuru and Kesari, 2008). Patients who exhibit a combination of ataxia and Lambert-Eaton syndrome against a background of small-cell lung cancer may have antibodies to voltage-gated calcium channels (VGCC). Cerebellar degeneration in Hodgkin disease often occurs months to years after the lymphoma presents and is associated with the anti-Tr antibody (Bernal et al., 2003). Less common antibodies that have been related to paraneoplastic cerebellar ataxia with or without additional signs include the anti-Ta, anti-Ma, and anti-CV2 antibodies and autoantibodies against metabotropic glutamate receptor and protein kinase C gamma (Hoffmann et al., 2008; Posner and Dalmau, 2000; Sabater et al., 2006; Sillevis et al., 2000). The zic4 antibody against the neuronal zinc-finger protein is also associated with small-cell lung cancer and ataxia (Bataller et al., 2004). When routine studies are unfruitful, positron emission tomography (PET) scan may be useful to detect small cancers (Land et al., 2005). The eradication of underlying cancer usually results in improvement or resolution of ataxia (Ammar et al., 2008). Plasmapheresis has been reported effective in the reduction of autoantibodies and improvement of symptoms (Armstrong et al., 2005; Bier and Hile, 2010; Hoffmann et al., 2008; Taniguchi et al., 2006).
Ataxia with Gluten Sensitivity
Gluten sensitivity is a systemic autoimmune disease with diverse manifestations. Celiac disease is only one aspect of this entity. Celiac disease is an autoimmune disorder of the small intestine caused by an immune reaction to gliadin, a prolamin (gluten protein) found in wheat. Its prevalence is between 1 in 1750 to 1 in 105 people in the United States, varying on the definition (Rewers, 2005). Cerebellar ataxia has been reported since 1966 in this group of patients (Cooke and Smith, 1966). Vitamin deficiency from malabsorption or concomitant autoimmune diseases may account for the ataxia. A more controversial area is the association of antigliadin antibodies in the etiology of sporadic ataxia with no overt evidence for small bowel disease.
Hadjivassiliou et al. (2010) reported that 47% (101/215) of patients with idiopathic sporadic ataxia had serological evidence of gluten sensitivity. Other studies have shown a lower prevalence of gliadin antibodies in patients with ataxia or none at all (Burk et al., 2001; Combarros et al., 2000; Pellecchia et al., 1999; Wong et al., 2007). Bushara et al. (2001) and Lock et al. (2005) reported that patients with idiopathic ataxia and those with definite genetic forms of ataxia had a similar occurrence of gluten sensitivity, raising doubts about the significance of the gliadin antibodies. Contrary data have been reported by Hadjivassiliou et al. (2003), noting that while there was a 14% prevalence of gluten sensitivity among inherited cases, the figure was 41% among 132 cases of sporadic ataxia. Gluten-free diet was shown to improve measures of ataxia over a 1-year period, associated with elimination of the antigliadin antibodies, but this was not a randomized study. Patients with gluten sensitivity usually have had slowly progressive ataxia associated with brisk tendon reflexes and peripheral neuropathy. Nervous system pathology was characterized by cerebellar Purkinje cell loss, infiltration by T lymphocytes and posterior column degeneration. A variable proportion of patients had typical celiac disease on duodenal biopsy, even when there were only minor gastrointestinal symptoms; some had either normal biopsies or simply lymphocytic infiltrates, so the role of gluten sensitivity in the etiology of sporadic ataxia remains controversial. Presence of antigliadin antibodies should prompt further testing including antideaminated gliadin antibody, antitransglutaminase antibody, and small-bowel biopsy. If celiac disease is confirmed, gluten-free diet may offer a good chance for symptomatic relief (Rashtak et al., 2010).
Ataxia and Anti–Glutamic Acid Decarboxylase Antibodies
A number of reports have noted the presence of antibodies to glutamic acid decarboxylase (GAD) among a small number of patients with progressive ataxia (Bayreuther et al., 2008; Honnorat et al., 1995; Rakocevic et al., 2006; Saiz et al., 1997). The patients have usually been middle-aged women. Ataxia was often associated with peripheral neuropathy, rarely associated with neuropathy or slow saccades and in some cases with stiff person syndrome. Many patients had multiple organ specific antibodies including those directed against thyroid, parietal cells, and pancreatic islet cells, and insulin-dependent diabetes was an invariable accompaniment. The antibodies occurred in higher titers than those found in adult-onset diabetes, and there is evidence that they bind to presynaptic nerve terminals around cerebellar Purkinje cells. GAD is the enzyme that synthesizes γ-aminobutyric acid (GABA) from glutamate, and it is believed that the antibodies may be pathogenic because of their binding to GABA terminals. Intravenous immunoglobulin or immunosuppressants may cause partial remission of symptoms (Markakis et al., 2008; Nanri et al., 2009; Nociti et al., 2010; Vulliemoz et al., 2007).
Superficial Siderosis
Superficial siderosis occurs when iron and hemosiderin are deposited in the pial and subpial regions of the brain. Clinical features include ataxia, hearing loss, and cognitive decline (Fearnley et al., 1995). Superficial siderosis results from bleeds that are often covert, such as with cryptic vascular malformations, or more obvious, such as with tumors or prior surgery. Diagnosis can be made by the characteristic hypointensity over CNS structures in T2-weighted images on MRI (Fig. 72.2). The disease can be controlled if the source of bleeding is found and obliterated.
Inherited Ataxias
The initial recognition that progressive balance difficulties related to pathology in the cerebellum and its connecting pathways could have a genetic basis is attributed to Nicholas Friedreich, who published a series of papers describing siblings with such a disease, now known as Friedreich ataxia (FA). As early as 1893, Pierre Marie recognized the clinical and genetic heterogeneity among the inherited ataxias. During much of the 20th century, inherited ataxias that did not quite fit the Friedreich mould were often labeled as Marie ataxia. It eventually became apparent that many patients have progressive ataxia of a degenerative nature, clinically and pathologically resembling patients with inherited ataxias, and yet have no discernible genetic basis. Much of the last 100 years saw detailed clinical and pathological descriptions of patients with various forms of ataxia and attempts at understanding them on a clinicopathological basis. The classification of these diseases based on the underlying neuropathology reflects attempts at such understanding. Nevertheless, the clinical, neuropathological, and genetic heterogeneity of the disorders did not allow a universally acceptable classification. Harding (1983), based on an extensive clinical study of many families, wrote a clinical-genetic classification that served as a prelude to an increasingly gene-based classification of the inherited ataxias (Harding, 1983). This section will summarize the current information regarding inherited forms of ataxia.
Autosomal Recessive Ataxias
As the autosomal recessive ataxias have been elucidated at the molecular genetic level, a semblance of a pathogenetic classification may be emerging, albeit subject to revisions or additions. The genetic abnormalities discovered so far have either pointed to abnormalities in mitochondria, oxidative stress, abnormalities of deoxyribonucleic acid (DNA) repair mechanisms, possibly abnormal protein folding, or changes in cytoskeletal proteins. Table 72.2 lists various autosomal recessive ataxias based on specific gene loci. Most of these diseases begin in childhood or early adult life; however, onset even late in life is possible with autosomal recessive ataxias. Singleton patients may occur in many families. Typically, parents do not manifest symptoms, since they are heterozygous for the mutation. If the sibship size is large, affected siblings may be found, and the disorder will affect both males and females. Parental consanguinity may be found but is not essential.
| Entity | Gene locus/gene | Mutation |
|---|---|---|
AOA1, Ataxia with oculomotor apraxia type 1; AOA2, ataxia with oculomotor apraxia type 2; ARCA1, autosomal recessive cerebellar ataxia type 1; ARSACS, autosomal recessive ataxia of Charlevoix-Saguenay; ATLD, ataxia-telangiectasia-like disorder; IOSCA, infantile-onset spinocerebellar ataxia; MIRAS, mitochondrial recessive ataxia syndrome; MSS, Marinesco-Sjögren syndrome; SCAN1, spinocerebellar ataxia with axonal neuropathy.
Friedreich Ataxia
Clinical Features
Friedreich ataxia is the most common inherited ataxia, with a prevalence of 1 in 50,000 Caucasians. Classical descriptions of the disease include an age at onset below 25 years, typically early in adolescence (Harding, 1981). Initial symptoms include gait difficulties and clumsiness. Neurological examination reveals gait ataxia, loss of proprioceptive sense in the lower limbs, and absence of deep tendon reflexes (Pandolfo, 2003). These findings are related to early pathology in dorsal root ganglion cells, and occasional patients may be mistaken for having a hereditary sensory neuropathy. However, most will exhibit signs indicating involvement of the CNS including dysarthria, some upper motor neuron findings such as extensor plantar responses, and eye movement abnormalities such as square-wave jerks. Rarely, patients may present with cardiac disease or a spinal deformity and are later found to develop neurological disease; they tend to lose ambulation in about 9 to 15 years. At this stage, patients have increasing ataxia of both upper and lower limbs, profound proprioceptive loss, areflexia, weakness of lower-limb muscles, flexor spasms, and increasing dysarthria and dysphagia. Optic atrophy and hearing loss may occur in a minority of patients.
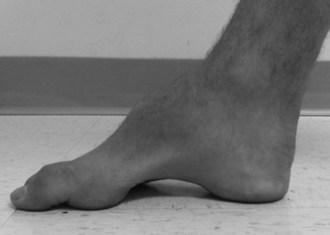
Systemic abnormalities include abnormal electrocardiograms, hypertrophic cardiomyopathy in about 50% of the patients, and diabetes in 10%. The hypertrophic cardiomyopathy may later become a dilated cardiomyopathy and rhythm problems such as atrial fibrillation may add to the cardiac morbidity. Skeletal abnormalities such as spinal deformities and foot deformities are common (Fig. 72.3). The mean age of death among patients with FA has been reported to be late in the fourth decade, but this information comes from clinical studies done prior to the availability of FA mutation analysis. Also, the availability of better diabetic and cardiac care as well as adaptive rehabilitation techniques can allow much longer survival. Cause of death is often cardiac, but respiratory compromise related to the motor difficulties and spinal deformity may also contribute.
Nerve conduction studies show early absence or reduction of sensory nerve potentials in a diffuse fashion, reflecting the loss of large sensory axons in peripheral nerves. Central motor conduction studies show abnormalities that evolve over the course of the disease and may reflect disease progression. MRI scans of the brain reveal no abnormalities in the cerebellum; rather, the upper cervical cord shows atrophy. Signal density changes may be seen in the posterior columns by MRI (Mascalchi et al., 1994). Functional imaging has been reported to show decreased cerebellar blood flow, but there is cerebral glucose hypermetabolism in ambulatory patients with FA (Gilman et al., 1990). Pathologically, there is loss of dorsal root ganglion cells, resultant degeneration of the dorsal columns, degeneration of spinocerebellar and corticospinal tracts, and loss of cells in the cerebellar dentate nucleus.
The Friedreich Ataxia Mutation
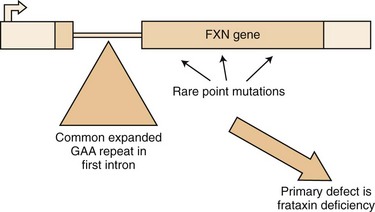
The mutation in FA is an unstable expansion of a repeated trinucleotide (GAA) sequence within the first intron of the FRDA gene on chromosome 9q13-21.1 (Campuzano et al., 1996) (Fig. 72.4). Expanded alleles have 66 to over 1000 repeats. In nearly 95% of affected persons, the GAA expansion occurs in both alleles (homozygous expansion), although the size of the expansion can be different in each.
Since the identification of the FA mutation, we have realized that many patients with a phenotype very atypical for classical FA carry the FA mutation (Durr et al., 1996; Filla et al., 1996; Montermini et al., 1997). Some 15% of patients with homozygous GAA expansion have neurological signs identical to those of classical FA but have an age at onset later than 25 years (late-onset FA [LOFA]). Other patients with the GAA expansion have the typical age at onset but retain their tendon reflexes or even have exaggerated reflexes (FA with retained reflexes [FARR]). There are patients who combine a later onset with retained reflexes as well; in addition, very late onset of mild gait ataxia, spastic paraparesis, and even chorea have been occasionally associated with the FA GAA expansion (Hou and Jankovic, 2003). The variation of expansion size may account for the clinical diversities. As with other trinucleotide repeat diseases, there is an inverse correlation between the size of the GAA repeat and the age at onset; this correlation is better with the smaller of the two expanded alleles. Cardiomyopathy and diabetes also tend to occur in patients with larger expansions (>700 GAA repeats). However, not all of the variation in age at onset and severity of disease can be correlated with GAA size alone. Other genetic and possibly environmental factors may contribute to this variation. In addition, variation in clinical picture can result from somatic mosaicism of the repeat size, with different tissues having significant differences in the size of the expansions (Bidichandani et al., 1999). Recently it has been reported that the U mitochondrial (mt)DNA haplogroup can delay the age at onset and have a lower rate of cardiomyopathy in FA (Giacchetti et al., 2004).
Point Mutations
Approximately 5% of patients with clinical findings compatible with FA have only one expanded allele; however, such patients harbor point mutations in the unexpanded allele. Sequencing of the unexpanded allele to document a point mutation will have to be done in this situation. Point mutations located in the carboxy terminal half of the mature frataxin appear to be associated with a typical FA phenotype (Cossee et al., 1999). Missense mutations in the amino terminal half of the protein, such as the G130V mutation, appear to result in a milder phenotype with less ataxia, greater spasticity, and no dysarthria. To date, all the patients with point mutations have been compound heterozygotes with one expanded allele. Homozygous point mutations will cause complete lack of frataxin, and this may be incompatible with life. This has been further supported by the fact that complete knockout of the gene in animal models is embryonically lethal.
Pathogenesis
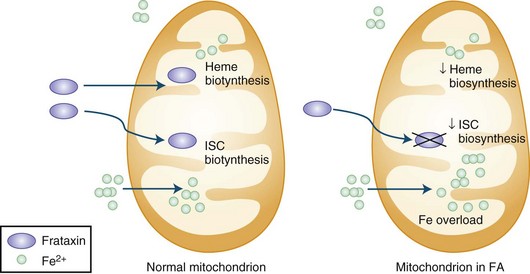
The presence of the expanded GAA sequence in the first intron of the gene results in reduced transcriptional efficiency leading to a partial deficiency of the protein, frataxin (Lodi et al., 2001; Pandolfo, 2001; Puccio and Koenig, 2000). The reduced transcriptional efficiency of the mutated gene has been attributed to an unusual “sticky DNA” configuration of the expanded repeat (Sakamoto et al., 1999). In addition, there is evidence for chromatin condensation and remodeling, which can further suppress frataxin transcription (Pandolfo and Pastore, 2009). The exact role of frataxin in normal biology is still unclear, but many studies suggest that it is a mitochondrial protein (Fig. 72.5). Knockout of the frataxin homolog in yeast leads to accumulation of iron in the mitochondria, a finding of interest in view of the presence of iron in the cardiac muscle in human disease (Babcock et al., 1997; Pandolfo and Pastore, 2009). Activity of enzymes such as aconitase and complex I, II, and III of the respiratory chain that contain iron-sulfur clusters is reduced in cardiac muscle biopsies of patients with FA (Rotig et al., 1997). The iron-sulfur cluster synthesis defect results from the role of frataxin in the process and probably is the cause of iron accumulation. It has been hypothesized that the presence of excess iron as well as excess oxidative stress related to impaired respiratory chain function can induce oxidative stress via the Fenton reaction and cause progressive nuclear and mitochondrial damage (Karthikeyan et al., 2002, 2003; Pandolfo and Pastore, 2009).
Treatment
Based on evidence for excess oxidative stress and poor defense mechanisms of FA tissue to such stress, antioxidant therapy has been tried over the last 10 years; as of 2008, at least 11 studies using idebenone in patients with FA have been reported (Meier and Buyse, 2009). This drug has been reasonably well tolerated in doses of 900 mg/day or more by FA patients, the main adverse effect being gastrointestinal. At doses of 5 mg/kg/day, it appeared to have beneficial effect on the cardiomyopathy of FA. In a 6-month study, idebenone given in high doses appeared to improve neurological function as measured by International Cooperative Ataxia Rating Scale (ICARS) scores in ambulatory FA patients (Schulz et al., 2009). Newer approaches now in the experimental phase include gene replacement (Fleming et al., 2005) and the use of molecules that may enhance frataxin production even in the presence of the GAA expansion (Hebert, 2008). In fact, recent open-label experience suggests some beneficial effect from erythropoietin (Boesch et al., 2008).
Mitochondrial Recessive Ataxia Syndrome
An autosomal recessive ataxic syndrome caused by a mutation in the polymerase γ (POLG) gene has been found to be the most common cause of recessive ataxia in Finland (Hakonen et al., 2005). Polymerase γ is encoded by the nuclear gene, POLG, in humans and is responsible for replication and repair of mtDNA. In a recent study report, 27 patients were found among 15 Finnish families with a carrier frequency of 1 : 125. In addition, patients with recessive ataxia from Norway, Belgium, and England shared the same core Finnish haplotype, suggesting a common European founder mutation and making it likely that this disease will be found in places other than Finland. The age at onset varied from 5 to 41 years. The neurological picture was heterogeneous, but ataxia, hypo- or areflexia, axonal neuropathy, and epilepsy were common. There was often mild cognitive impairment and psychiatric manifestations such as depression, anxiety, and hallucinations.
Infantile-Onset Spinocerebellar Ataxia
Infantile-onset spinocerebellar ataxia has been described among an isolated population in Finland. The disorder begins soon after children acquire the ability to walk. Neurological signs include ataxia, peripheral neuropathy with areflexia, athetosis of hands and feet, Babinski signs, and clumsiness. Speech becomes impaired, and sensorineural hearing loss occurs before the age of 5. Ophthalmoplegia and optic atrophy develop, and some patients have seizures. Patients usually have learning deficits and skeletal deformities as well. The gene responsible has recently been found to be C10ORF2, a nuclear gene encoding for Twinkle, an mtDNA-specific helicase, and a splice variant known as Twinky (Nikali et al., 2005) located on 10q24. Dominant mutations in the same gene cause a syndrome of progressive external ophthalmoplegia. In contrast to the latter, the recessive mutations in infantile-onset spinocerebellar ataxia do not appear to alter mtDNA maintenance.
Ataxia with Isolated Vitamin E Deficiency
The occurrence of a childhood-onset recessive ataxia associated with isolated vitamin E deficiency is related to mutations in the gene encoding the α-tocopherol transfer protein (α-TTP) on chromosome 8 (Ouahchi et al., 1995). A hepatic protein, α-TTP is involved in the processing of vitamin E for transport in the chylomicrons. The low vitamin E levels are related to impaired hepatic processing, not to deficient intestinal absorption. Deficiency of vitamin E, which has antioxidant properties, may cause neuronal degeneration. Purkinje cells are more vulnerable owing to their high metabolic activity and high oxygen demand. The severity of the phenotype associated with α-TTP mutations depends on residual protein activity. The childhood-onset disease has considerable resemblance to FA. There is progressive ataxia and evidence for a polyneuropathy with loss of proprioception and tendon reflexes. Retinitis pigmentosa and visual loss can accompany this syndrome. Patients often have head titubation. Cardiomyopathy can occur but is less common than in FA (Cavalier et al., 1998). Vitamin E levels should be obtained in all persons with sporadic ataxia of childhood or young-adult onset. Patients typically have less than 1.8 mg/L of vitamin E. Treatment with large doses of vitamin E stabilizes the neurological features, especially when started early in the disease, but some features may show worsening despite therapy (Mariotti et al., 2004).
Abetalipoproteinemia
Abetalipoproteinemia results from mutations in the MTTP gene, which codes for a subunit of the microsomal triglyceride transfer protein; this results in impaired lipid absorption in the intestine, including lipid-soluble vitamins like vitamin E. The ataxia resembles that in isolated vitamin E deficiency but is associated with evidence for malabsorption (Fogel and Perlman, 2007). Vitamin E and cholesterol levels are low, and apolipoprotein B is absent. Acanthocytes are found in peripheral blood smears. Transaminases may be high and INR elevated due to vitamin K deficiency. Serum lipoprotein electrophoresis can establish the diagnosis. Treatment involves dietary modification and vitamin replacement, which may prevent neurological complications.
Cayman Ataxia
This recessive ataxia of early childhood onset has been identified in the Grand Cayman island. Affected patients have been shown to carry two homozygous mutations in the gene that encodes caytaxin (ATCAY) on chromosome 19. Caytaxin contains a region with homology to a CRAL-TRIO domain, also present in α-TTP. A ligand similar to vitamin E may bind to this protein, and its deficiency may cause ataxia by a mechanism similar to that in vitamin E deficiency (Bomar et al., 2003).
Ataxia-Telangiectasia
Clinical Features
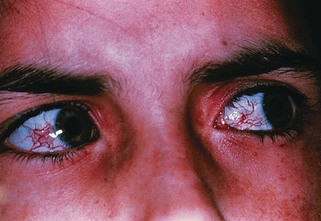
The occurrence of ataxia-telangiectasia (AT) has been estimated to be 1 in 20,000 to 100,000 live births (Swift et al., 1993). This disorder has its onset early in the first decade with truncal instability and impaired gait. This is followed by progressive ataxia associated with polyneuropathy, hypotonia, and loss of reflexes (Fogel and Perlman, 2007). Choreiform movements are frequent. Characteristic eye movement abnormalities are seen, with the need for a rapid head thrust for fixating on targets to one side, with the eyes following later (eye-head lag). This is referred to as oculomotor apraxia. Telangiectasia tends to appear at about 5 years of age and is most frequent on the conjunctivae (Fig. 72.6) but can also occur over the earlobes, popliteal fossa, and other locations. There is evidence for an immunodeficiency, with frequent bronchopulmonary infections. These children are at risk for malignancies, especially hematological tumors like lymphomas and leukemias; other types of cancers occur if the patient reaches adulthood. Infections related to immunodeficiency are also common. The thymus gland is typically small or absent in patients with AT. Many have decreased immunoglobulin (Ig)A levels; decreased IgE and IgM levels, lymphocytopenia, and skin anergy may also occur. In addition, elevation of α-fetoprotein (AFP) in the serum is a consistent finding in AT.
Mutation and Pathogenesis
AT is caused by truncating, missense, deletion, or insertion mutations of the ATM gene on chromosome 11 (Halazonetis and Shiloh, 1999; Savitsky et al., 1995). More than 300 mutations of this gene have been associated with AT. The protein product of ATM is a serine-threonine kinase and may be involved in checkpoint responses to double-stranded DNA breaks by conveying the signal of damage from sensor proteins such as MRN (MRE11-RAD50-NBS1 complex) to the effector proteins. Increased susceptibility to radiation damage has been shown to cause chromosomal translocations and has been used in a fibroblast survival assay as a diagnostic test. The degree of DNA damage determines the response: either cell-cycle arrest and repair or initiation of apoptosis (Lavin et al., 2005).
Laboratory Diagnosis
Serum AFP is usually elevated (>10 ng/mL), and immunoglobulin levels are low. Karyotyping often shows chromosomal abnormalities, especially a 7 : 14 translocation. Increased sensitivity of cultured fibroblasts to radiation can be documented by the colony survival assay. The definitive way of establishing diagnosis is immunoblotting for the ATM protein; 90% of patients have no protein, 10% have trace amounts, and rare patients have normal ATM levels but no kinase activity (so-called kinase-dead) (www.geneclinics.org). Further molecular confirmation can be achieved by detecting the mutation, but this can be difficult because of the size of the ATM gene and the lack of mutation “hot-spots.” Sequence analysis of the coding regions of the ATM gene will detect 90% of mutations but miss intronic alterations and heterozygous deletions; benign polymorphisms and pathogenic alterations may be difficult to distinguish. Other types of DNA-based tests include protein truncation tests (examining for premature stop codons), which can detect about 70% of the mutations, and mutation scanning using denaturing high-performance liquid chromatography (DHPLC), which has a sensitivity of 85%.