Diabetes Mellitus and Pregnancy
History
Before the discovery of insulin, pregnancy in a woman with DM was little more than a medical curiosity. The few women with DM who survived adolescence were often infertile. Those who conceived frequently underwent therapeutic abortion in view of the alarmingly high rates of both maternal (25%) and perinatal (40% to 50%) mortality present at the time. After therapy with insulin became available, women with diabetes generally reached adulthood with little impairment in fertility. Maternal mortality declined to a rate similar to that of women without DM. A comparable reduction in fetal wastage did not occur until much later. In the 1950s and 1960s, pioneering efforts based on the premise that fetal survival is linked to control of maternal diabetes reduced the rates of fetal loss to 10% to 15%. Further improvements followed the development of technologies for (1) monitoring the integrity of the fetoplacental unit, (2) documenting maternal metabolic control more accurately (i.e., self-monitoring of capillary blood sugar), and (3) sophisticated management of neonatal morbidity. In centers that regularly provide specialized team care to substantial numbers of patients, rates of perinatal loss in diabetic pregnancies (except for those related to major congenital malformations) now approach those of the general obstetric population. Thus attention has increasingly focused on neonatal morbidity and the potential effects of maternal diabetes on the offspring in later life. For a historical perspective, see the comprehensive Technical Review published recently by the American Diabetes Association (ADA).1
In recent years, increasing numbers of women with long duration of type 1 DM are having pregnancies sometimes in the presence of vascular and/or neuropathic complications. In the past decade, the prevalence of preexisting type 2 DM complicating pregnancy has increased throughout the world. Rates of congenital malformations and adverse pregnancy outcome tend to be as high as those in pregnancies complicated by type 1 DM.2
Criteria for the diagnosis of GDM were initially established over 40 years ago3 and with minor modifications remain in widespread use today. These criteria were chosen to identify women at high risk for development of diabetes following pregnancy, not to identify pregnancies at increased risk for adverse perinatal outcomes. Others use criteria for GDM that are the same as those used to classify glucose tolerance in nonpregnant persons.4 Consequently, for about 3 decades, much controversy has existed regarding the medical/obstetrical significance of GDM and the cost effectiveness of its detection and treatment. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study5 has reported strong continuous associations of maternal glucose levels below those diagnostic of diabetes with increased birth weight and increased cord-blood serum C-peptide levels (fetal hyperinsulinemia). Significant associations were also found with the two other primary outcomes (primary cesarean section delivery and clinically defined neonatal hypoglycemia) and with several secondary outcomes (premature delivery [<37 weeks’ gestation], preeclampsia, shoulder dystocia or birth injury, need for intensive neonatal care, or hyperbilirubinemia), but these tended to be weaker. It is anticipated that in the near future, these results and other data will stimulate the adoption of outcome-based criteria for diagnosis and classification of states of hyperglycemia in pregnancy.
Epidemiology
Over the past 2 decades, the age-adjusted prevalence of DM has increased in the general population of women of reproductive age.6 In 1995, based on data from the 1988 National Maternal and Infant Health Survey (NMIHS), Engelau et al.7 reported a 4% prevalence of diabetes complicating pregnancy in the United States. They estimated that GDM accounted for 88% and preexisting DM 12% of cases. Type 2 DM (NIDDM) accounted for two-thirds and type 1 DM (IDDM) one-third of preexisting DM. Studies reported recently provide evidence that the prevalence of both GDM and pregnancy in women with preexisting DM has increased since the NMIHS data were collected.8 For example, the age- and race/ethnicity–adjusted prevalence of preexisting diabetes in pregnancy increased from 0.81% in 1999 to 1.82% in 2005 in the population participating in the Kaiser Permanente Southern California Medical Care Program. Preexisting diabetes represented 10% of deliveries with any form of diabetes complicating pregnancy in 1999. By 2005, it represented 21%. Furthermore, the greatest proportional increase in preexisting DM was found in the youngest cohort, aged 13 to 19 years. Information was not sufficient to distinguish between preexisting type 1 and type 2 DM; however, ethnic/racial distribution and other demographic characteristics suggest that the increase in preexisting DM in pregnancy is primarily in type 2 DM.8 In contrast, the prevalence of GDM, which had been increasing, (see later) did not change during this 6-year interval.
Using a database from the Kaiser Permanente Northern California Medical Care Program that employed the same screening and diagnostic criteria throughout the study period, Ferrara et al.9 found an increase in the incidence of GDM from 1991 to 1997 (from 5.1% to 7.4%) and leveling of the rate from 1997 to 2000. An increase was found in all racial/ethnic groups of the heterogeneous population. In Colorado, Dabelea et al.10 found that the prevalence of GDM doubled between 1994 and 2002 in the population served by Kaiser Permanente, but the absolute prevalences were lower than reported from Northern California. Here also, the same screening and diagnostic criteria were followed throughout the period of observation, but they required higher levels of glucose for the diagnosis of GDM than those used by Ferrara et al.9,11,12 This alone would lead to differences in observed frequency of GDM. In another recent publication based on the National Hospital Discharge Survey (NHDS) of births in the United States between 1989 and 2004, Getahun et al.13 also reported an increase in the prevalence of GDM among all racial and ethnic groups. However, in this report, the largest relative increase was found among blacks. There is also a general impression that GDM prevalence has increased globally14; however, aside from the two reports mentioned above, these impressions are not based on standardized procedures and universal testing of populations.
Pathogenesis
Metabolic Effects of Pregnancy
The metabolic alterations that develop during pregnancy are profound, but they do not occur with equal intensity throughout gestation. Rather, a temporal progression is seen in which increasing insulin resistance and other metabolic changes parallel the growth of the conceptus. In the immediate postpartum period, the profound insulin resistance dissipates rapidly. These metabolic perturbations and their temporal associations suggest that they derive from the conceptus.15 Serial estimates of insulin sensitivity both before and during pregnancy in a relatively small number of women with normal carbohydrate metabolism indicate a slight reduction in insulin sensitivity by 12 to 14 weeks and a further decline by the end of the second trimester.16 During the third trimester insulin sensitivity is 40%-60% lower than in nongravid women.16–19 Catalano and colleagues16 found modest improvement in insulin sensitivity at 12 to 14 weeks in women with GDM when compared with their state of insulin resistance before pregnancy. This modest improvement was followed by progression to severe insulin resistance in late gestation that was equal to or greater than that in subjects with normal glucose tolerance. Women with type 1 DM who are in optimal metabolic control before conception do not have an increase in insulin requirement during the first trimester and may even require some reduction in dosage because of hypoglycemia at the end of the first and beginning of the second trimester (Fig. 30-1).20
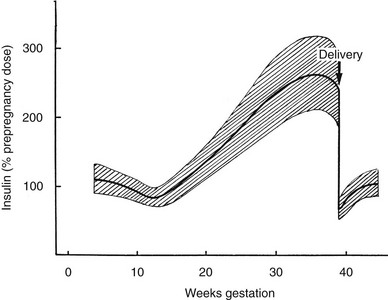
FIGURE 30-1 Schematic representation of changing insulin requirements over the course of pregnancy and after delivery in pregestational diabetes mellitus. (Data from Phelps RL, Metzger BE, Freinkel N: Medical management of diabetes in pregnancy. In Sciarra J (ed): Gynecology and obstetrics, vol 3, Philadelphia, 1988, Harper & Row, pp 1–16.)
In early pregnancy, there is little if any increase in insulin secretion in response to glucose. Conversely, insulin secretion in response to oral or intravenous glucose in the last trimester of pregnancy is approximately 1.5 to 2.5 times greater than that seen in nongravid conditions15 and is accompanied by islet cell hyperplasia. The product of β-cell secretion is primarily insulin and not a disproportionate amount of proinsulin or intermediates, which have substantially less activity than insulin. Insulin does not cross the placenta. Although the human placenta is small in proportion to total maternal mass, it actively degrades insulin and moderately increases insulin clearance in normal pregnancy and GDM.19,21
These changes occur temporally in parallel with increasing size of the placenta and growth of the fetus. However, the specific mediators of increased insulin secretion and insulin resistance are not entirely clear. Table 30-1 lists a number of the many factors potentially implicated in these changes. Numerous studies suggest that progesterone, acting either separately or in concert with estrogens, has direct β-cell cytotropic actions. When the two sex steroids are administered to nonpregnant animals in appropriate molar concentration ratios, effects on plasma insulin and fuel storage in liver and adipose tissue similar to those seen in normal pregnancy are observed without significantly affecting skeletal muscle sensitivity to insulin.22 Higher circulating concentrations of maternal leptin, potentially of placental origin,23 may reflect the change in insulin sensitivity rather than directly contributing to it. During the latter half of pregnancy, circulating levels of human chorionic somatomammotropin (hCS) or placental lactogen, estrogen, and progesterone reach maximal plasma concentrations with increasing placental mass.15 The concentration of pituitary growth hormone decreases,24 but the increasing level of the growth hormone variant (hGH-V) of placental origin may offset the decline.24 Prolactin also increases throughout gestation and may contribute to the insulin resistance. Free cortisol levels increase, but the diurnal variations are maintained25 despite the presence of placental corticotropin and corticotropin-releasing factor. In recent years, several other factors derived from the placenta and/or adipose tissue have been identified as potentially important contributors to insulin resistance in normal pregnancy and GDM. These include increases in tumor necrosis factor α (TNF-α)26 and decreases in adiponectin.27 Several other factors that potentially contribute to insulin resistance in type 2 DM have not been fully evaluated in normal pregnancy or GDM.28
Table 30-1
Factors of Placental Origin That May Influence Maternal Insulin Sensitivity
Estrogens and progesterone
Human chorionic somatomammotropin (hCS) or placental lactogen (HPL)
Prolactin
Placental growth hormone variant (hGH-V)
Corticotropin-releasing factor (CRF) and corticotropin
Leptin
Tumor necrosis factor α (TNF-α)
Adiponectin*
Resistin
Ghrelin
Interleukin 6 (IL-6)
*There is controversy about whether the placenta is or is not a source of adiponectin.
In a recent review, Friedman and colleagues29 concluded that at the molecular level, the insulin resistance of normal pregnancy is multifactorial, involving reduced ability of insulin to phosphorylate the insulin receptor, decreased expression of insulin receptor substrate 1 (IRS-1), and increased levels of a specific kinase. Further changes occur in GDM that inhibit signaling and lead to substantially reduced GLUT4 translocation. The net effect of these combined hormonal and metabolic changes is to oppose insulin action at peripheral (muscle and adipose tissue) and hepatic sites.
Utilization of Maternal Fuels by the Conceptus
The placenta is the conduit through which the conceptus continuously draws maternal fuel for its metabolic and biosynthetic needs, and glucose is the major source of its metabolic energy. In addition, glucose or three-carbon intermediates derived from glucose (lactate) are precursors for glycogen, glycoproteins, and the glyceride-glycerol in triglycerides and phospholipids of the conceptus. Glucose utilization rates as high as 6 mg/kg/min have been estimated in the human fetus at term,30 in contrast to glucose turnover of 2 to 3 mg/kg/min in normal adults. Glucose delivery across the placenta occurs by facilitated diffusion, and maternal glucose usually exceeds fetal glucose concentration by 10 to 20 mg/dL (0.6 to 1.1 mmol/L).
In the third trimester, growth of the human fetus requires the net placental transfer of approximately 54 mmol of nitrogen per day.31 Furthermore, amino acids may be used in the conceptus for oxidative energy. Although quantitative measurements of nitrogen requirement for fetal growth in humans are not available, it is clear that the fetus exerts an unremitting drain on maternal nitrogen reserves.
Although maternal triglyceride represents the largest reserve fuel depot, it can directly support the metabolic needs of the conceptus only to a limited extent. Triglycerides cross the placental barrier poorly, and the net transfer of free fatty acids (FFAs) to the fetus may be limited. Glycerol can cross the placenta readily, but its contribution in nonruminant mammalian species is probably small. Ketones readily cross the placenta, are present in the fetal circulation in concentrations approaching those in maternal blood,32 and the enzymes necessary for ketone oxidation are present in the human fetus. When fetal tissues, including the brain, are incubated in vitro with concentrations of ketones similar to those present during fasting, substantial oxidation of ketones is seen, even in the presence of alternative fuels (i.e., fasting concentrations of glucose, lactate, and amino acids).32 Oxidation of ketones lessens that of the other fuels and may spare them for biosynthetic disposition or other pathways in the fetus.33 However, such diversion to the metabolism of ketones may have adverse consequences. Ketones inhibit pyrimidine and purine synthesis in developing brain cells in the rat fetus33 and at high concentrations disrupt organogenesis in rodent embryos in culture. Controversial epidemiologic evidence suggested that maternal ketonuria during human pregnancy may be associated with reduction in the intelligence quotient (IQ) of the offspring in childhood.34 Rizzo and co-workers35 reported an inverse association between increased plasma FFAs and β-hydroxybutyrate concentrations in the second and third trimesters of pregnancy and intellectual development of offspring at age 2 to 5 years.
Circulating Concentrations of Nutrient Fuels
In Normal Pregnancy: Normal women have a decrease in the concentration of fasting plasma glucose (FPG) during pregnancy. The greatest decline in FPG (10- to 12-hour fast) occurs early in gestation,36 well before the rate of glucose utilization by the fetus is sufficient to increase total maternal glucose turnover. It has been reported that severely obese women do not show a decline of FPG during pregnancy.36 A lower FPG persists during late gestation despite relatively higher postmeal glucose levels. However, reports of diurnal glucose profiles of ambulatory pregnant women obtained by capillary blood glucose monitoring or continuous monitoring of subcutaneous fluid confirm that glycemic excursions vary within a narrow range in normal subjects, even during late gestation.37,38 Basal concentrations of plasma glycerol and FFAs do not change until late gestation, at which time significant elevations occur, and transition to the metabolic profile characteristic of the fasting state is accelerated in association with mounting lipolysis and insulin resistance.39 Progressive increases occur in all major lipid fractions, including triglycerides, cholesterol, and phospholipids.22 Total plasma amino acid concentrations also decline in early pregnancy and persist throughout gestation.40 The reasons for these changes are not clear. The suppressive effects of insulin on plasma amino acids are well known and could account for this finding. However, in later gestation, release of amino acids from skeletal muscle is less restrained by insulin, at least in pregnant rats. This suggests that during this time of insulin resistance, increased fetal removal, as opposed to impaired muscle release, may play a primary role in sustaining maternal hypoaminoacidemia.
In Gestational Diabetes Mellitus: Basal and postprandial levels of glucose, FFAs, triglycerides, and amino acids tend to exceed those of normal pregnant control subjects,41 and the changes tend to persist during dietary intervention, with the extent of the abnormalities paralleling the severity of the GDM.41 Branched-chain amino acids are sensitive to insulin, are often altered in obesity and other insulin-resistant states, and are the most consistently disturbed.41 The propensity to “accelerated starvation” (e.g., a more rapid decline in circulating glucose concentration in association with a greater increase in FFAs and ketones) in women with GDM is similar to that found in women with normal glucose homeostasis.42 Diurnal glucose profiles of ambulatory women with diet-treated GDM obtained by continuous monitoring of subcutaneous fluid show greater glycemic excursions and delay in reaching postprandial peak values than seen in normal subjects.38
In Women With Preexisting Diabetes Mellitus: In pregnant women in whom type 1 DM is well controlled, few disturbances in plasma lipids (FFAs, cholesterol, and triglycerides) have been found, and individual lipoprotein fractions have little change in their lipid content.43 The greatest departures from the norm during pregnancy occur in plasma glucose profiles; plasma amino acid concentrations also may be markedly disturbed. Changes in amino acids and indices of glycemic control (blood glucose self-monitoring records and hemoglobin A1c levels) are poorly correlated, especially in late pregnancy.44 Lipids tend to be altered more extensively in pregnant women with type 2 DM, with higher total plasma triglycerides and an increased triglyceride content of very-low-density lipoproteins.43 The cholesterol content of high-density lipoproteins may be decreased when compared with levels in normal pregnancy or in pregnant women with type 1 DM.43 The relative roles of obesity and diabetes in the development of these lipid aberrations remain to be defined. Studies of amino acid metabolism in type 2 DM in pregnancy have not been reported.
Maternal Metabolism and Pregnancy Outcome
The pioneering hypothesis advanced by Pedersen45 a half century ago stated that maternal hyperglycemia leads to fetal hyperinsulinism, which is responsible for macrosomia and neonatal morbidity. Extensive experimental and clinical evidence indicates that metabolic disturbances in the mother contribute to virtually all the adverse effects of DM on the offspring.15,46,47 The importance of alterations in other metabolic fuels, in addition to glucose, was recognized later.41 Results of the HAPO Study5 indicate that the associations between maternal glycemia, fetal insulin, and parameters of fetal growth extend through the full range from “normal” to those that reflect overt diabetes. Freinkel15 emphasized the temporal relations between a metabolic insult and the adverse outcome expected (“fuel-mediated teratogenesis”) and postulated that the altered intrauterine environment of diabetes can have lifelong as well as perinatal consequences.15,46 The key features of the hypotheses of Pedersen and Freinkel are schematically integrated in Fig. 30-2.
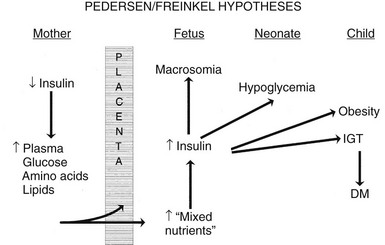
FIGURE 30-2 Effect of maternal fuels on fetal development. The classic hyperglycemia-hyperinsulinemia hypothesis of Pedersen45 has been modified to show the contribution of other insulin-responsive maternal fuels besides glucose. All of these fuels can influence the growth of the fetus and the maturation of its insulin secretion. As indicated here, altered fetal nutrients and enhanced insulin secretion are associated with consequences that extend well beyond the neonatal period. (Data from Silverman BL, Purdy LP, Metzger BE: The intrauterine environment: Implications for the offspring of diabetic mothers, Diabetes Rev 4:21–35, 1996.)
Congenital Malformations and Early Fetal Loss
Increased risks of congenital malformations and spontaneous abortions in diabetic pregnancies are linked to metabolic control at conception.15,47 Good control during the period of organogenesis may reduce the prevalence of these adverse outcomes.47 Risk of spontaneous abortion increases in direct proportion to hemoglobin A1c concentration measured shortly before or after conception.48,49 The specific relation between metabolic control and risk of congenital malformations has been more difficult to define. Greene and associates49 found a prevalence of congenital malformation of about 5% until initial hemoglobin A1c concentrations were in excess of 10 to 12 SD of the mean control value. If put in the context of current analytical methods and reference range for pregnancy,50 this would represent hemoglobin A1c in the range of 9.5 to 10%. Beyond that, the risk of malformations increased steeply. Several groups reported that improving control of DM before conception51 reduces rates of major congenital malformations to those expected in the general obstetric population. In populations in which most pregnancies in women with diabetes are planned, congenital malformations have declined to rates similar to those of the general population.52 However, data from general population–based sources indicate that the overall risk of birth defects remains nearly 10% in pregnancies in women with preexisting diabetes.53,54
In vivo and in vitro animal models suggest that diabetic embryopathy is multifactorial.55 Oxidative stress, increased generation of free radicals, disruption of signaling pathways, including the expression and action of the Pax3 gene, or a combination of these have been implicated.53,54,56 When tight metabolic control is restored, levels of circulating serum factors that may mediate embryopathy decline more slowly than hyperglycemia and hyperketonemia.55 Hypoglycemia also is potentially teratogenic,57 so measurements of blood glucose or hemoglobin A1c may not fully reflect the “toxicity” of the maternal environment for the fetus. This lack of specificity is reflected in the fact that 60% to 70% of offspring of mothers with first-trimester hemoglobin A1c levels indicative of poor metabolic control are normally developed at birth.49 Consequently, neither the precise degree of glycemic control nor the interval over which good control must be maintained to achieve optimal outcome is known.47
Disturbances of Fetal Growth
Development of macrosomia (traditionally defined as birth weight > 4000 g or above the 90th percentile for gestational age) is the quintessential fulfillment of the Pedersen hypothesis and a frequent complication of pregnancies complicated by DM and GDM. Increased adiposity is the primary component of the macrosomia. Infants of diabetic mothers may have up to twice the body-fat content of infants of normal mothers. Increased fat content was reported in infants of mothers with GDM, even with total body weight identical to that of controls,58 and data from the HAPO Study5 showed that the risk of high infant percent body fat increased in association with higher maternal glucose concentration across the entire range of subdiabetic glucose levels. Adiposity tends to be prominent in the shoulder region, enhancing risks for cesarean delivery, shoulder dystocia, and birth trauma.59 Skin-fold measurements may be used to document adiposity at birth and reflect maternal metabolic regulation.60 However, skin-fold measurements are difficult to standardize and are seldom used in routine clinical assessment.60
Asymmetric growth is one hallmark of diabetic fetopathy. In addition to hypertrophy of subcutaneous fat, other organs responsive to insulin (e.g., the heart and liver) may be larger, whereas insulin-insensitive tissues such as the brain are of normal size. Thickness of fetal humeral soft tissue61 or cheek-to-cheek dimensions62 can be used to detect asymmetric growth caused by maternal diabetes. Some investigators use ultrasound-measured abdominal circumference that is greater than the 75th percentile to identify pregnancies at higher risk for macrosomia and target them for intensive therapy with insulin.63
Fetal hyperinsulinemia may develop early in gestation, well before adipose tissue develops.64 Morphometric studies of the pancreas from fetuses of mothers with diabetes demonstrated islet hypertrophy and hyperplasia during the second trimester. We observed a stronger association between fetal islet function near term or at birth and metabolic control in the second trimester (hemoglobin A1c concentration) than in the third trimester.65 Once initiated, β-cell overactivity may promote development of macrosomia, even without sustained elevations in nutrient fuels. Visceromegaly and fat accumulation also resulted from insulin administration to normal fetal monkeys (via implanted insulin pumps), without concurrent infusion of additional nutrients.66 The results of the HAPO Study indicate that the risk of fetal hyperinsulinemia increases in a linear fashion across the range of maternal glucose concentrations below those characteristic of overt diabetes.5 Alterations of multiple nutrient fuels65 may contribute to premature activation of fetal islet function and increases in insulin-like growth factors (IGFs).67
Historically, intrauterine growth restriction (IUGR) was a common finding in offspring of type 1 diabetic mothers. This was thought to be secondary to maternal vascular disease, resulting in uteroplacental insufficiency.45 However, in early pregnancy very poor metabolic control (in the absence of vasculopathy) may retard growth irreparably, even without associated birth defects.1 At the present time, growth restriction is rarely seen with diabetes except in pregnancies complicated by hypertension or nephropathy.
Anthropometric and Metabolic Development in Childhood
Pettitt and colleagues68 found a correlation in Pima Indian mothers between the 2-hour response to oral glucose during pregnancy and the occurrence of obesity in their offspring. Moreover, the risk of obesity was not limited to those whose birth weight was increased.69,70 Greater relative weight for height was reported at age 4 years in the offspring of diabetic mothers whose control was “poor” rather than “good” during the index pregnancy.69 In a prospective long-term follow-up study of offspring of diabetic mothers, our group at Northwestern University found that macrosomia of offspring disappeared by age 1 year. However, by age 8 years, obesity was highly prevalent; nearly half had a weight greater than the 90th percentile.69,71 Recently, Hillier and colleagues reported weight of children (ages 5 to 7 years) from a large multiethnic group cohort whose mothers had glucose challenge tests and/or glucose tolerance tests during pregnancy.72 Risk of obesity in the children increased progressively across the range of subdiabetic maternal glucose values.
In Pima Indians, by age 20 to 24 years, type 2 DM is present in 45.5% of offspring of “diabetic” mothers, 8.6% in offspring of “prediabetic” mothers, and 1.4% of offspring of “nondiabetic” mothers.73 The differences remain after adjustment for diabetes in the father, age at onset of diabetes in either parent, and obesity in the offspring (Fig. 30-3). The authors concluded, “The findings suggest that the intrauterine environment is an important determinant of the development of diabetes and that its effect is in addition to effects of genetic factors.”73 The offspring of diabetic mothers enrolled in the Northwestern University long-term follow-up had a high prevalence of impaired glucose tolerance (IGT),74 particularly during puberty. IGT developed at similar rates in the offspring of mothers with GDM and preexisting DM. Excessive insulin secretion in utero was a strong predictor of both IGT and obesity in childhood, independent of degree of obesity.74 Together, these observations indicate that in offspring of diabetic mothers, nature (genetic factors) and nurture (intrauterine metabolic environment) may interact in predisposing to obesity and type 2 DM.
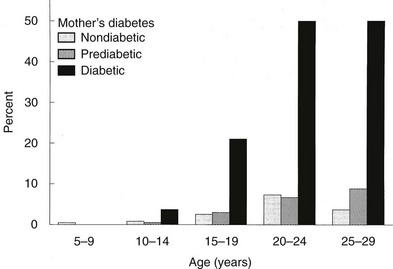
FIGURE 30-3 Age-specific prevalence of non–insulin-dependent diabetes mellitus (plasma glucose > 200 mg/dL 2 hours after oral glucose) in offspring of Pima Indian women without diabetes mellitus (light gray bars), those developing diabetes only subsequent to pregnancy (hatched bars), or those with diabetes during pregnancy (solid bars). (Data from Pettitt DJ, Aleck KA, Baird HR et al: Congenital susceptibility to NIDDM: Role of intrauterine environment, Diabetes 37:622–628, 1988.)
Diagnosis and Classification
We advocate the scheme outlined in Table 30-2 for the classification of carbohydrate intolerance during pregnancy. It incorporates many of the recommendations of an American Diabetes Association expert committee12 and those of the Fourth11 and Fifth75 International Workshop Conferences on GDM.
Table 30-2
Classification of Glucose Metabolism
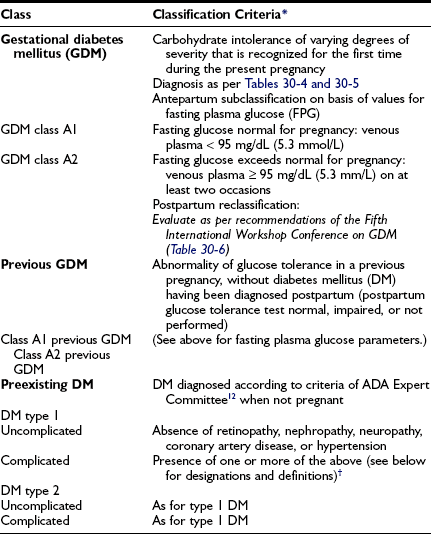
*Classification is based on prevailing practice in the authors’ center and the recommendations of the American Diabetes Association’s Expert Committee and the Fourth and Fifth International Workshop Conferences on Gestational Diabetes.11,12,75
†Designations and definitions of DM types 1 and 2 (designations are appended to primary diagnosis as appropriate [e.g., “diabetes mellitus type 2 uncomplicated”; “diabetes mellitus type 1; BDR, NEPH”]): BDR, Background diabetic retinopathy; CAD, coronary artery disease diagnosed by history, electrocardiogram (ECG), or stress ECG; HTN, hypertension, defined as blood pressure ≥ 140/90 mm Hg consistently; NEPH, diabetic nephropathy, defined as ≥ 0.5 g protein in 24-hr urine collection and/or serum creatinine consistently ≥ 1.2 mg/dL (≥106 mol/L); NEUR, neuropathy, defined as known gastroparesis when not pregnant, orthostatic hypotension, or sensory abnormalities in lower extremities detected at bedside examination; PDR, proliferative diabetic retinopathy.
Gestational Diabetes Mellitus
GDM is subclassified to distinguish between those with FPG values within the normal range for pregnancy (i.e., < 95 mg/dL [5.3 mmol/L]) and those with values exceeding the limits of normal (i.e., ≥ 95 mg/dL [5.3 mmol/L]). Those with higher FPG are at greater risk for progress to a diagnosis of diabetes outside of pregnancy,76 and some have arbitrarily initiated pharmacologic therapy in those with elevated FPG in the diagnostic OGTT.77 Patients are often seen who had GDM in a previous pregnancy but had no postpartum evaluation of glucose metabolism. Others may have had impaired fasting glucose or IGT postpartum, but not DM. From the perspective of proper classification and epidemiology, the diagnosis of GDM should not be assigned to such patients in a subsequent pregnancy. However, for purposes of clinical management, it is appropriate to stratify them on the basis of FPG (see earlier) and to designate them as GDM class A1, previous GDM, or as GDM class A2, previous GDM.
Preexisting Diabetes
We subdivide patients with preexisting DM into those with presumed type 1 or type 2 DM. Historically, the White classification78 was devised to predict pregnancy risk in type 1 DM based on age at onset and duration of diabetes, in combination with microvascular or macrovascular complications. In the present era, fetal loss is uncommon, and the degree of metabolic control throughout pregnancy and the presence or absence of vascular complications, independent of maternal age or duration of DM, are more specific predictors of maternal or fetal morbidity. Therefore, we currently use the White classification only for descriptive purposes. To assist in the estimation of maternal and fetal risks, we designate pregnancies in women with DM as uncomplicated (no known vascular or neuropathic complications) or complicated (one or more complications). Abbreviations for the specific complication(s) are added as a postscript. Hare79 proposed a similar although not identical classification.
Physicians would like to provide pregnant women who have complications of diabetes prospective answers to two questions: Will pregnancy accelerate or worsen preexisting complications? Does the diabetic complication per se contribute to the risk of adverse pregnancy outcome? In many cases, evidence is not sufficient to offer specific advice. These complicated issues have been reviewed in detail in a recent technical report from the American Diabetes Association.43 In the next several paragraphs, we comment on those situations for which the greatest amount of specific information is available.
Retinopathy: Diabetic retinopathy may worsen during gestation. The risk is present primarily in women with active proliferative changes or severe preproliferative retinopathy. Patients with mild background retinopathy or inactive laser-treated proliferative disease rarely experience progression of consequence. An association has been found between worsening retinopathy during pregnancy and the severity of hyperglycemia at enrollment80,81 and the magnitude of improved glycemic control achieved in the first half of gestation.80 This worsening during pregnancy may be analogous to the transient deterioration observed in nonpregnant subjects after the initiation of “tight” control of diabetes.82 Data from the Diabetes Control and Complications Trial82 indicate that pregnancy per se adds independently to the risk of transient progression of retinopathy, and the increased risk of progression may continue during the first postpartum year. Hypertension in pregnancy also is associated with progression of diabetic retinopathy.83 Regardless of the mechanisms involved, women with preexisting retinopathy should be advised of the potential for deterioration and the need for close ophthalmologic follow-up before conception, during pregnancy, and in the postpartum period. Although photocoagulation therapy can be used effectively during gestation, those with active proliferative disease should be advised to postpone pregnancy until photocoagulation treatment has stabilized the retinal condition.
Nephropathy: Diabetic nephropathy (24-hour urine protein ≥ 0.5 g or reduced creatinine clearance) increases risks for both the mother and offspring.43,84 Worsening proteinuria (twofold to threefold increase), hypertension, premature labor, and a need for early induction are common outcomes. The risks of these complications increase with stage of nephropathy (Table 30-3). Most women experience little permanent effect on renal function, despite transient but substantial increases in proteinuria.84,85 Occasionally, patients experience deterioration in renal function that continues in the postpartum period.43 Whether this decline is related to pregnancy or reflects the natural progression of renal impairment is uncertain. The number of subjects with severe diabetic nephropathy is too small to gain definitive information at any single center.
Table 30-3
Stages of the Evolution of Diabetic Nephropathy and Common Effects on Pregnancy
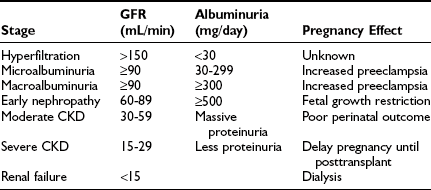
CKD, Chronic kidney disease; GFR, glomerular filtration rate.
Data from Ref. 43, p 375.
Neuropathy: Diabetic neuropathy is commonly found in patients with longstanding diabetes. Little is known about the effect of pregnancy on progression of diabetic neuropathy. However, autonomic neuropathy may contribute to maternal morbidity and adverse pregnancy outcome.43,86,87 Gastroparesis may result in marked glucose lability, inadequate nutrition, and maternal pulmonary aspiration. Bladder dysfunction may increase risk for urinary tract infection and worsening renal function.
Cardiovascular Disease: Both systolic and diastolic blood pressure may increase in pregnancy in type 1 diabetic women.88 In dated studies, myocardial infarction was associated with 50% mortality.89,90 An increased risk for myocardial infarction and congestive heart failure is also found in the postpartum period. The number of subjects with either longstanding type 1 or type 2 DM who experience coronary artery disease during pregnancy is small. At this time, an efficient, cost-effective strategy for detection and treatment of cardiovascular disease before and during pregnancy is not available.43
Diagnosis of Gestational Diabetes Mellitus
The optimal cost-effective strategy for the detection and diagnosis of GDM has been the subject of much controversy for decades. In the United States and a number of other countries, the standard procedure is to do a screening blood glucose (50-gm glucose challenge test [GCT]) followed by a 3-hour OGTT in those with a positive GCT. In some other countries, an OGTT is performed as the only blood glucose test in women with a history of GDM risk factors. It is anticipated that translation of the results of the HAPO Study5 and the increasing prevalence of GDM9,10 (see Epidemiology section) will lead to a less complicated screening and diagnostic algorithm for all pregnant women that will be widely if not globally adopted. Previously diagnosed and undiagnosed type 2 DM have also increased in prevalence8 and are generally asymptomatic. Testing blood glucose concentrations in all women at the first obstetric appointment and serially throughout pregnancy is not cost effective in most populations. However, those with undiagnosed DM or IGT before conception have hyperglycemia in the first trimester and may benefit from early diagnosis and initiation of therapy. Therefore, it is important to have a comprehensive strategy for GDM diagnosis that is tailored to the population served. Participants in the Fourth and Fifth International Workshop Conferences on Gestational Diabetes Mellitus recommended that an assessment of risk for GDM as outlined in Table 30-4 be performed during the first prenatal visit.11,75
Table 30-4
Screening Strategy for Detecting Gestational Diabetes Mellitus11,75
GDM Risk Assessment Should Be Ascertained at the First Prenatal Visit
High risk: Perform blood glucose testing as soon as feasible if one or more present:
If GDM is not diagnosed, blood glucose testing should be repeated at 24–28 weeks or at any time a patient has symptoms or signs suggestive of hyperglycemia.
Average risk: Perform blood glucose testing at 24–28 weeks by using either:
• Two-step procedure: 50-gm glucose challenge test (GCT) followed by a diagnostic oral glucose tolerance test (OGTT) in those meeting threshold value in GCT (see text for details) or
• One-step procedure: Diagnostic OGTT performed on all subjects (see text for details)
Low risk: Blood glucose testing not routinely required if all of these characteristics are present:
• Member of an ethnic group with a low prevalence of GDM







