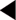CHAPTER 5 DIABETES COMPLICATIONS
EYES
RATIONALE
Diabetes has long been associated with eye problems, including retinopathy, cataracts and glaucoma. Individually or collectively, these problems can lead to visual loss. Diabetic retinopathy is a specific vascular complication of diabetes and is the most common cause of newly registered cases of blindness (12%) in those aged 20 to 64 years in the UK (Evans et al 1991). In type 2 diabetics, glaucoma and cataracts are a more frequent cause of visual loss than retinopathy, but the latter is already present at diagnosis in one-fifth of patients (Macleod et al 1994) and its prevalence relates directly to the duration of diabetes.
Among the risk factors associated with the development and progression of diabetic retinal disease in type 2 diabetics are: raised blood pressure (UKPDS 1998c), increased duration of diabetes (Klein et al 1984), and the presence of microalbuminuria (Savage et al 1996).
The St Vincent Declaration of 1989 set a target to reduce new blindness caused by diabetes by one-third or more (Krans et al 1995). Regular accurate screening for diabetic eye disease can improve the prognosis by detecting abnormalities earlier in their natural history. Active management of microangiopathy affecting the retina has been possible since the development of laser photocoagulation. Cataract surgery and other surgical techniques that may benefit patients with diabetic eye disease are now available.
TARGETS
The aims of a screening and management programme for diabetic eye disease should be to:
SCREENING
Programmes
The two preferred methods of screening for diabetic retinopathy that meet the National Clinical Guidelines’ recommendation of a sensitivity of at least 80% and a specificity of at least 95% (Hutchinson et al 2001) are:
The National Screening Committee recommended that a diabetic retinopathy screening programme should:
The preferred screening methods are more likely to meet these criteria.
Fundoscopy
The findings of the examination will determine the classification of diabetic retinopathy, as outlined in Figure 5.1.
INTERVENTIONS
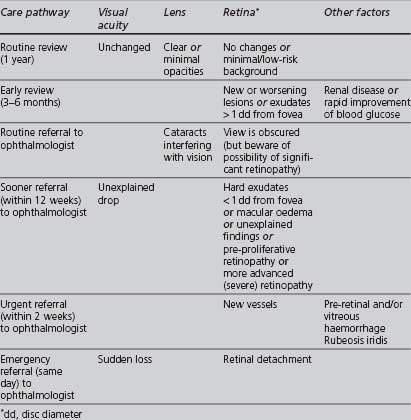
The appropriate management of diabetic retinopathy should follow the pathway outlined in Table 5.1.
TABLE 5.1 Management pathways following eye assessment (NICE 2002a, UK National Screening Committee 2004)
Suitable interventions that can be undertaken in primary care include:
Secondary-care interventions may include the following:
NEPHROPATHY
RATIONALE
Diabetic nephropathy is one of the more serious complications of diabetes. It occurs in 20–40% of patients with diabetes and its prevalence is increasing. Indo-Asian and Afro-Caribbean type 2 diabetics are at a greater risk of developing diabetic nephropathy than whites. In England, the rates for initiating treatment for end-stage renal disease were reported as 4.2 and 3.7 times higher in Afro-Caribbeans and Indo-Asians, respectively, than in whites (Roderick et al 1996).
Persistent albumin excretion greater than 300 mg/day, termed macroalbuminuria, in the absence of infection, marks the onset of diabetic nephropathy, and is also associated with increased mortality, particularly from vascular causes (Macleod et al 1995). The natural history of overt diabetic nephropathy is to progress to end-stage renal failure. The presence of lesser amounts of urinary albumin (less than 300 mg/day), termed microalbuminuria, indicates early renal damage. This is a risk factor for cardiovascular morbidity and mortality in type 2 diabetics (Dinneen & Gerstein 1997). About half of diabetics develop microalbuminuria at some stage. Of these (the study’s subjects were on insulin) 30–50% will progress to macroalbuminuria, but 30–50% will revert to normal albumin excretion and 20–30% will continue to have microalbuminuria (Laing et al 2003). If retinopathy is also present, then diabetes-related renal disease is the likely cause of the albuminuria. If retinopathy is absent, other causes of renal disease should be considered (NICE 2002b).
Prompt and effective interventions, particularly at an early stage, may both prevent end-stage renal failure and reduce the high risk of cardiovascular disease. The management of chronic kidney disease is now the subject of an NSF published in 2005 (DH Renal NSF Team 2006).
SCREENING
Screening for diabetic nephropathy should check at least annually two parameters:
Urinary albumin (including microalbuminuria)
The categorisation of levels of albumin excretion is explained in Table 5.2.
| Category | Spot collection (ng/ml creatinine) |
|---|---|
| Normal | < 30 |
| Microalbuminuria | 30–299 |
| Macro- (clinical) albuminuria | 300 or more |
The “gold standard” for the measurement of urinary albumin excretion is a timed urine sample (either over 24 hours, enabling simultaneous measurement of creatinine clearance, or 4 hours), but this is not a practical screening procedure for widespread use in the community.
Sending an early morning urine sample (also referred to as a “spot check”) to a chemical pathology laboratory to measure the albumin:creatinine ratio (ACR) is both more practical and the preferred method recommended by the ADA. ACR levels equal to or greater than 2.5 mg/mmol in males or 3.5 mg/mmol in females indicate microalbuminuria. The test needs to be repeated for confirmation, as microalbuminuria can be transient. One of the authors completed a study in which the prevalence of microalbuminuria in diabetics in an ethnically mixed UK community was 19.3%. The characteristics independently associated with a higher prevalence of microalbuminuria were current insulin use, current smoking, older age, higher systolic blood pressure and poorer metabolic control, but there was no significant association with either increasing duration or gender. Unfortunately, the sample size was not large enough to determine whether there was an association between microalbuminuria and Indo-Asian ethnicity (Levene et al 2004).
Estimated glomerular filtration rate
The glomerular filtration rate can be calculated using a formula and has been proposed as a more accurate, but logistically feasible, estimation of renal function. The 4-variable Modification of Diet and Renal Disease (MDRD) (Levey et al 1999) formula uses the patient’s creatinine, age (valid only between 18 and 70 years), sex and race:
Pathology laboratories have started reporting eGFR automatically (with white ethnicity as default) using the MDRD formula: the result must be multiplied by 1.21 if the patient is black African. If the laboratory does not provide an eGFR result or if looking at a historical creatinine result, then eGFR can be calculated using a readily available online calculator: http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm.
The Renal NSF classifies those with kidney disease into 5 stages, based upon eGFR and the presence of urinary albumin (see Table 5.3).
TABLE 5.3 Classification into CKD stages 1 to 5, based upon estimated glomerular filtration ratio (eGFR)
| Stage | eGFR (ml/min) | Other |
|---|---|---|
| 1 | ≥ 90 | Haematuria or proteinuria must be present |
| 2 | 60–89 | Haematuria or proteinuria must be present |
| 3 | 30–59 | |
| 4 | 15–29 | |
| 5 | ≤ 15 or renal replacement therapy |
MANAGEMENT
A number of interventions have been shown to delay the progression of diabetic nephropathy.
Management can be divided into nonpharmacological and pharmacological:
Nonpharmacological
Pharmacological
Lowering blood pressure
Tight blood pressure control is the most important intervention for delaying the development of diabetic nephropathy (UKPDS 1998c). The target is a diastolic blood pressure below 75 mmHg in “normotensive insulin-dependent” or 90 mmHg in “hypertensive non-insulin-dependent” (BNF).
There is strong evidence for the reno-protective effect of ACE inhibitors/ARBs which have a greater effect upon urinary albumin excretion, a stronger marker for renal disease and cardiovascular health, than other blood-pressure-lowering drug classes. A Cochrane review suggests that ACE inhibitors are the best drug class to prevent microalbuminuria and nephropathy (Strippoli et al 2005). One randomised controlled trial (RCT), whose subjects were people with type 2 diabetes, hypertension, and microalbuminuria, reported that, compared with placebo, an ARB (irbesartan) reduced progression from early to late nephropathy over 2 years (Parving et al 2001). Another RCT, whose subjects were people with type 2 diabetes and early nephropathy found no significant difference in glomerular filtration rate change, mortality, or cardiovascular disease (CVD) events between an ARB (telmisartan) and an ACE inhibitor (enalapril) over 5 years (Barnett et al 2004). Other studies have found ARBs to be reno-protective in patients with type 2 diabetes and overt nephropathy (Brenner et al 2001, Lewis et al 2001).
However, effective blood pressure control is paramount, and non-DCCBs, β-blockers, or diuretics should be used in combination with or, if ACE inhibitor/ARB is contraindicated or not tolerated, as alternatives to inhibition of the renin-angiotensin-aldosterone system (Black et al 2003, Pepine et al 2003).
Improving lipid profile
Prescribing a statin reduces cardiovascular risk in patients with diabetic nephropathy.
The small Danish Steno-2 study compared the effect of a targeted, intensified, multifactorial (both lifestyle and pharmacological) intervention with that of conventional treatment on modifiable risk factors for CVD in type 2 diabetics and microalbuminuria, showing significantly reduced hazard ratios for the development of both macro- and microvascular complications in type 2 diabetics with microalbuminuria (Gaede et al 2003).
FEET
See also the subsection on neuropathy.
RATIONALE
Definition
WHO has defined the diabetic foot as a group of syndromes in which neuropathy, ischaemia and infection lead to tissue breakdown, resulting in morbidity and possible amputation (Krans et al 1995).
Pathological processes
There are two main pathological pathways in diabetic feet:
In diabetics attending dedicated foot clinics, approximately 50% have neuropathic feet and 50% have neuro-ischaemic feet (Edmonds et al 1986).
The burden of diabetic foot problems
A UK population-based study in type 2 patients gave a prevalence of 1.4% for foot ulcers, but the prevalence of the risk factors that give rise to ulcers was 41.6% (Kumar et al 1994). Once a limb has been amputated the prognosis for the contralateral limb is poor (Ebskov & Josephsen 1980). Foot ulcers occur more frequently in whites than in Indo-Asians or Afro-Caribbeans, and are associated with adverse social circumstances such as deprivation and isolation (Boulton 1997), poor glycaemic control, the presence of other vascular risk factors (e.g. smoking) and increased duration of diabetes.